Difference between revisions of "DCOM Volume I Appendix I"
| (2 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | = | + | =APPENDIX I: MEASUREMENTS OF WATER HARDNESS= |
'''Hardness and its Measurement'''<br> | '''Hardness and its Measurement'''<br> | ||
| − | Hardness is expressed in terms of mg/l by weight in terms of calcium carbonate. Water | + | Hardness is expressed in terms of mg/l by weight and in terms of calcium |
| + | carbonate. Water with hardness not exceeding 70 mg/l is termed ‘soft’ and | ||
| + | above that 'hard'. In public water supplies, it used to be customary to reduce | ||
| + | carbonate hardness to 35 - 40 mg/l and total hardness to between 50 and | ||
| + | 100 mg/l. However as indicated above this is no longer recommended unless | ||
| + | hardness exceeds about 130 mg/l, but should still be practised for strictly | ||
| + | industrial supplies of water. | ||
| − | '''Hardness = | + | '''Hardness = Σ divalent cations = Ca<sub>2</sub>+ + Mg<sub>2+</sub> + Fe<sub>2+</sub> + Mn<sub>2+</sub> + Sr<sub>2+</sub>..'''<br> |
| + | The principle cations causing hardness in water and major anions associated | ||
| + | with them are as follows: | ||
| − | + | [[Image:AppendixITable1.PNG|780px|link=DCOM_Volume_I]] <br> | |
| − | Table I. | + | If hardness is too high it results into precipitation of soap, scaling on pipes, |
| + | boilers, cooling towers, heat exchangers. If hardness is too low, the water | ||
| + | becomes corrosive. Hard water interferes with almost every cleaning task from | ||
| + | laundering and dishwashing to bathing and personal grooming (IANR, Water | ||
| + | Quality 1996). Hard water ranges between 120-250 mg/L as CaCO3 or beyond | ||
| + | 250 mg/L as CaCO3 for very hard waters. The acceptable water hardness range is | ||
| + | between 60-120 mg/L as CaCO3 (Dipa Dey, Amanda Herzog and Vidya Srinivasan, | ||
| + | 2007). The scale of hardness is shown in Table I.2: | ||
| − | + | [[Image:AppendixITable2.PNG|780px|link=DCOM_Volume_I]] <br> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | '''Temporary and Permanent Hardness'''<br> | |
| − | + | Hardness can be described as temporary or permanent as shown in the Table: | |
| − | + | I.3 Carbonate hardness is called temporary because it precipitates readily at | |
| + | high temperatures since it is sensitive to heat and precipitates readily at high | ||
| + | temperatures. Therefore, hardness can be removed by boiling the water. Noncarbonate | ||
| + | hardness is called permanent because it does not precipitate readily | ||
| + | at high temperatures. | ||
| − | + | [[Image:AppendixItable3.PNG|780px|link=DCOM_Volume_I]] <br> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | Previous Page: [[DCOM_Volume_I_Appendix_H|DCOM_Volume_I_Appendix_H]] << >> Next Page: [[DCOM_Volume_I_Appendix_J|DCOM_Volume_I_Appendix_J]] | |
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 16:26, 2 June 2021
APPENDIX I: MEASUREMENTS OF WATER HARDNESS
Hardness and its Measurement
Hardness is expressed in terms of mg/l by weight and in terms of calcium
carbonate. Water with hardness not exceeding 70 mg/l is termed ‘soft’ and
above that 'hard'. In public water supplies, it used to be customary to reduce
carbonate hardness to 35 - 40 mg/l and total hardness to between 50 and
100 mg/l. However as indicated above this is no longer recommended unless
hardness exceeds about 130 mg/l, but should still be practised for strictly
industrial supplies of water.
Hardness = Σ divalent cations = Ca2+ + Mg2+ + Fe2+ + Mn2+ + Sr2+..
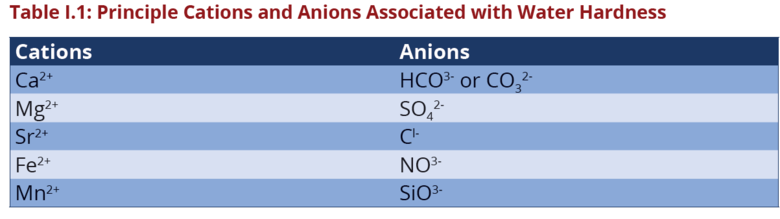
The principle cations causing hardness in water and major anions associated
with them are as follows:
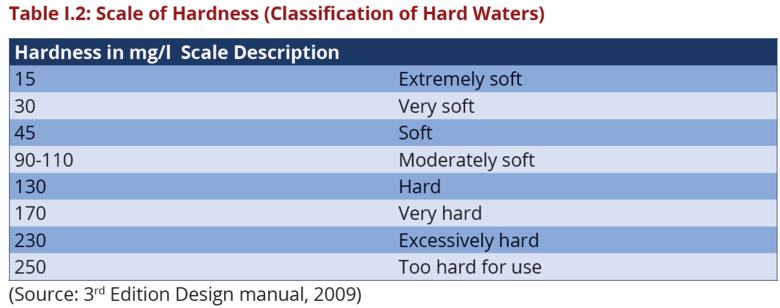
If hardness is too high it results into precipitation of soap, scaling on pipes, boilers, cooling towers, heat exchangers. If hardness is too low, the water becomes corrosive. Hard water interferes with almost every cleaning task from laundering and dishwashing to bathing and personal grooming (IANR, Water Quality 1996). Hard water ranges between 120-250 mg/L as CaCO3 or beyond 250 mg/L as CaCO3 for very hard waters. The acceptable water hardness range is between 60-120 mg/L as CaCO3 (Dipa Dey, Amanda Herzog and Vidya Srinivasan, 2007). The scale of hardness is shown in Table I.2:
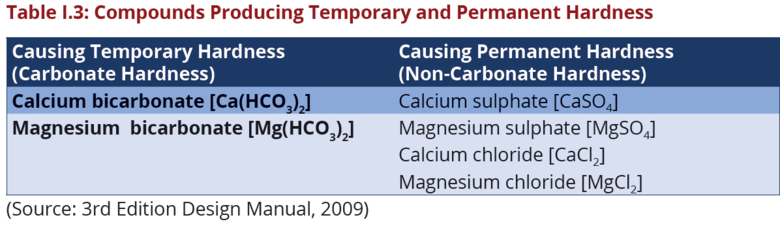
Temporary and Permanent Hardness
Hardness can be described as temporary or permanent as shown in the Table:
I.3 Carbonate hardness is called temporary because it precipitates readily at
high temperatures since it is sensitive to heat and precipitates readily at high
temperatures. Therefore, hardness can be removed by boiling the water. Noncarbonate
hardness is called permanent because it does not precipitate readily
at high temperatures.
Previous Page: DCOM_Volume_I_Appendix_H << >> Next Page: DCOM_Volume_I_Appendix_J