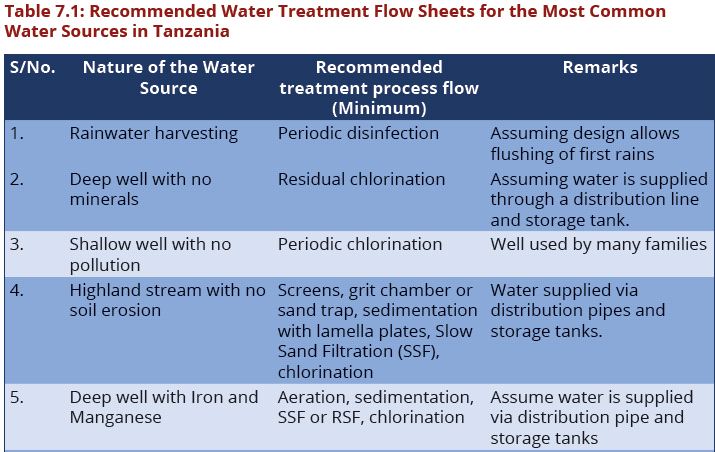
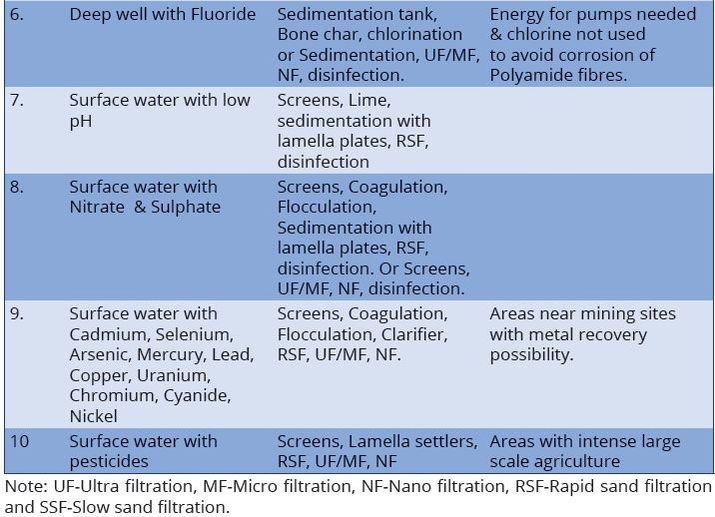
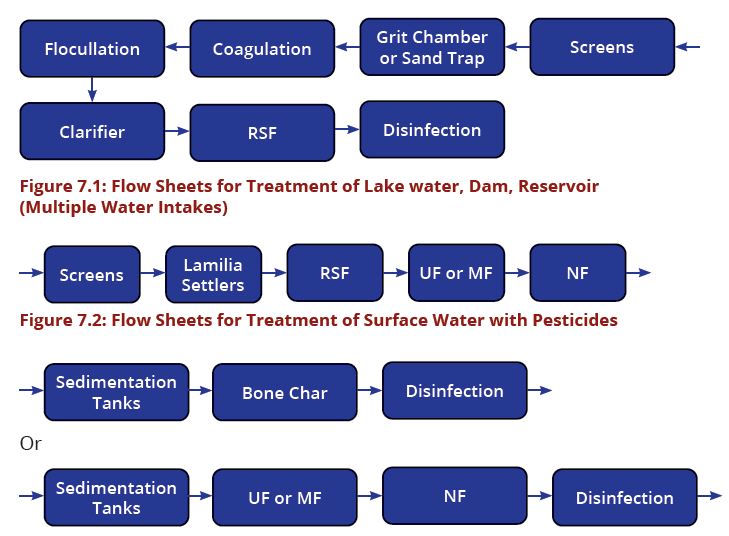
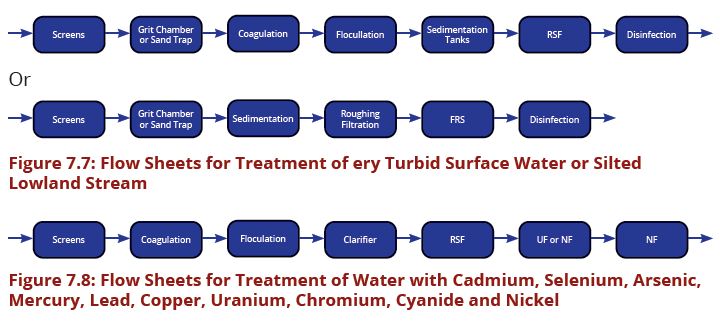
Difference between revisions of "Chapter Seven: Water Treatment"
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
= Chapter 7: WATER TREATMENT = | = Chapter 7: WATER TREATMENT = | ||
| − | + | <div style="text-align: justify"> | |
==INTRODUCTION == | ==INTRODUCTION == | ||
In this chapter, different categories of the unit operations that are utilized to achieve different water treatment levels are described. It is followed by description of the recommended approach of design of treatment plant components. Emphasis should be given to potential water sources that have undergone investigations on the variability of both the quality and quantity for at least two years. The data gathered should be used for selection of appropriate treatment flow sheets and designing it. | In this chapter, different categories of the unit operations that are utilized to achieve different water treatment levels are described. It is followed by description of the recommended approach of design of treatment plant components. Emphasis should be given to potential water sources that have undergone investigations on the variability of both the quality and quantity for at least two years. The data gathered should be used for selection of appropriate treatment flow sheets and designing it. | ||
| Line 146: | Line 146: | ||
==== Sedimentation ==== | ==== Sedimentation ==== | ||
| − | In designing sedimentation tanks, the required detention time determines the dimensions of the tank. A rectangular tank is the simplest design to use. Detention time is calculated as Volume/Flow rate (Q). The detention | + | In designing sedimentation tanks, the required detention time determines the |
| − | + | dimensions of the tank. A rectangular tank is the simplest design to use. Detention | |
| − | + | time is calculated as Volume/Flow rate (Q). The detention time based on the | |
| − | + | average daily flows usually ranges from about 45 minutes to 3 hours depending | |
| − | + | on water turbidity. The ideal inlet reduces the entry velocity and distributes the | |
| − | + | water as uniformly as possible across the depth and width of the tank. Outlets are | |
| − | + | usually weirs that are sufficiently long to reduce the flow velocity, and so avoid | |
| − | + | the re-suspension of the solids in the water. Plain sedimentation tanks should be | |
| − | + | designed for a surface loading in the range of 0.1 - 0.5 m3/m2/h. The exact surface | |
| − | + | loading to be adopted should be determined after carrying out settlement tests | |
| − | + | on samples of raw water, typical of all regimes of the water source. The settling | |
| − | + | properties of water will depend on the soil and vegetation conditions in the | |
| − | + | catchment area, and they will vary considerably between different locations and | |
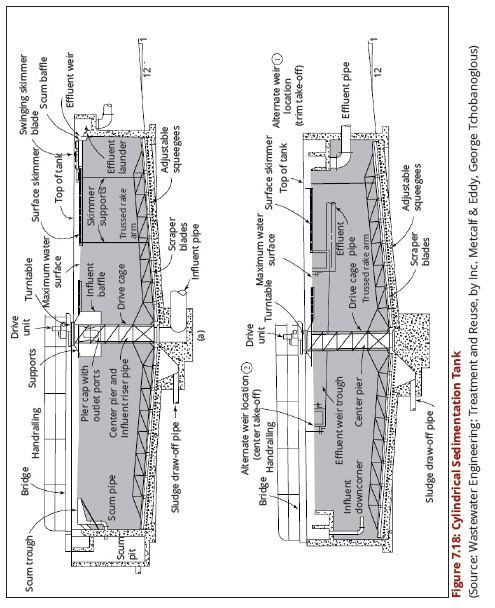
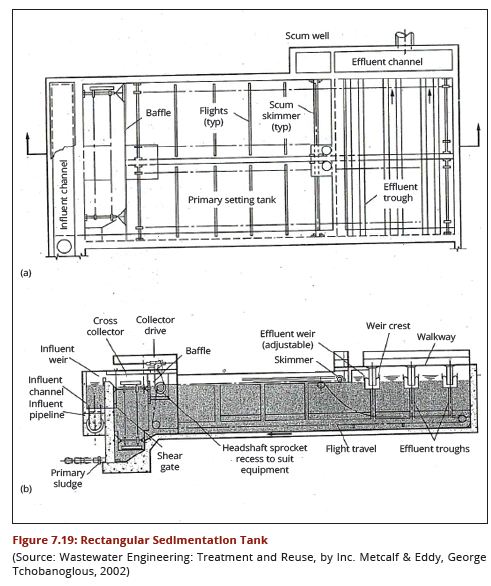
| − | + | regimes of the water source. Figures 7.18 (a and b) show a cross-section through | |
| − | + | a circular sedimentation tank while Figures 7.19 (a) and (b) show a cross-section | |
| + | through a rectangular sedimentation tank. | ||
==== Lamella Plate Settlers (Inclined plate settlers) ==== | ==== Lamella Plate Settlers (Inclined plate settlers) ==== | ||
| Line 167: | Line 168: | ||
– 60o) continuous gravity drainage of the settleable material onto the floor below can be achieved, without impairment of effluent quality. However, to work effectively an efficient flocculation stage is critical. | – 60o) continuous gravity drainage of the settleable material onto the floor below can be achieved, without impairment of effluent quality. However, to work effectively an efficient flocculation stage is critical. | ||
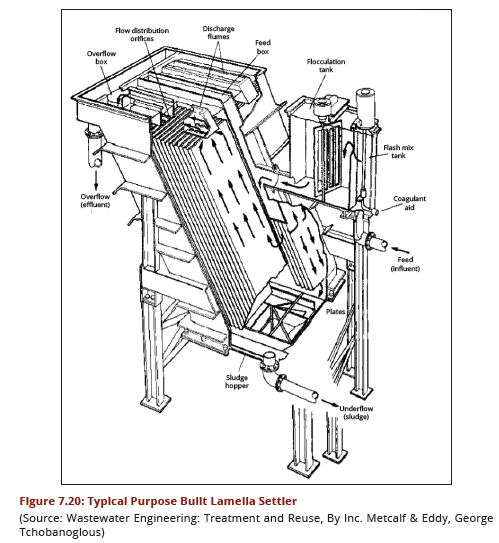
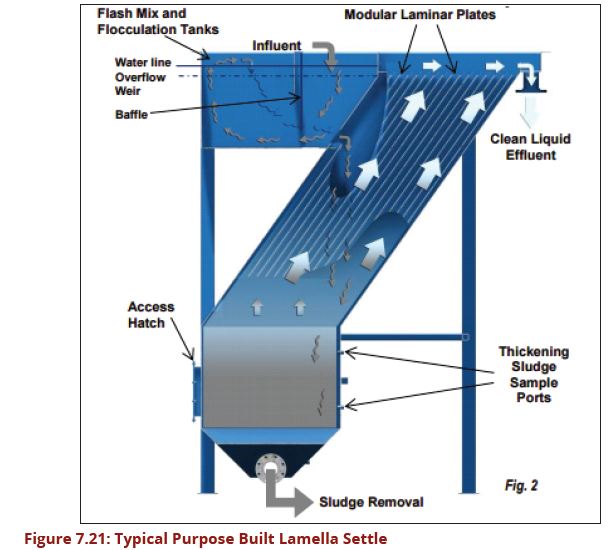
| − | In purpose built lamella plate settlers the water enters at the base of the lamella plates and travels upwards between the lamellas. Each space between the lamella plates | + | In purpose-built lamella plate settlers, the water enters at the base of the lamella plates and travels upwards between the lamellas. Each space between the lamella plates tends to act as a semi-independent settling module with the lamella plates extending from near the base of the tank to about 125 mm above the top water level. The clarified water is collected by saw-toothed notched launders running along each side of the plate. Unless sludge is removed mechanically by a scraper, sufficient depth beneath the plates is required for access during cleaning, although this can be aided by pressurized water. A typical arrangement is illustrated in Figure 7.20. |
| + | |||
| + | [[Image:Figure7.18.JPG|500px|link=Chapter_Seven:_Water_Treatment]] | ||
| + | |||
| + | [[Image:Figure7.19.JPG|491px|link=Chapter_Seven:_Water_Treatment]] | ||
| + | |||
| + | top water level. The clarified water is collected by saw-toothed notched launders | ||
| + | running along each side of the plate. Unless the sludge is removed mechanically | ||
| + | by a scraper, sufficient depth beneath the plates is required for access during | ||
| + | cleaning, although this can be aided by pressurized water. A typical arrangement | ||
| + | is illustrated in Figure 7.20. | ||
| + | |||
| + | [[Image:Figure7.20.JPG|499px|link=Chapter_Seven:_Water_Treatment]] | ||
| − | + | [[Image:Figure7.21.JPG|614px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Plates are made of stainless steel or plastics with a width of 1.25 to 1.5 m and a length of 2.5 to 3.25 m including the length above water. It is advisable to use standard lengths of metal sheets and plastic sheets available in the market. In Tanzania sheets are in dimensions of 120 cm x 240 cm. Plate thickness is usually about 0.7 mm for stainless steel whilst the horizontal spacing between plates is varied according to the nature of the raw water but within the range 50 – 80 mm. | Plates are made of stainless steel or plastics with a width of 1.25 to 1.5 m and a length of 2.5 to 3.25 m including the length above water. It is advisable to use standard lengths of metal sheets and plastic sheets available in the market. In Tanzania sheets are in dimensions of 120 cm x 240 cm. Plate thickness is usually about 0.7 mm for stainless steel whilst the horizontal spacing between plates is varied according to the nature of the raw water but within the range 50 – 80 mm. | ||
| Line 188: | Line 196: | ||
A = Settled area (m<sup>2</sup>) | A = Settled area (m<sup>2</sup>) | ||
| − | + | [[Image:Table7.2a.JPG|644px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | | | ||
| − | | | ||
| − | |||
| − | |||
| − | |||
| − | + | [[Image:Figure7.22.JPG|603px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
==== Primary Filtration ==== | ==== Primary Filtration ==== | ||
| − | Primary filtration of water removes material | + | Primary filtration of water removes material whose particle size is greater than |
| + | the opening size. The target particles are those which are not removed by | ||
| + | sedimentation tanks. | ||
==== Slow Sand Filtration ==== | ==== Slow Sand Filtration ==== | ||
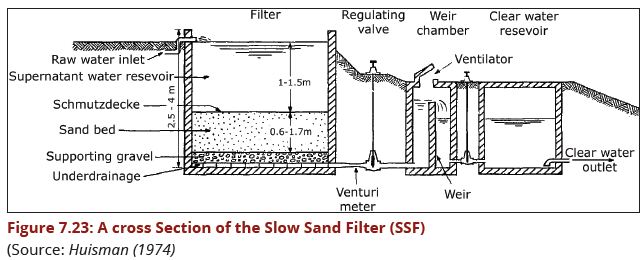
| − | A Slow Sand Filter (SSF) is basically a large tank containing the sand bed. A distinguishing feature of slow sand | + | A Slow Sand Filter (SSF) is basically a large tank containing the sand bed. A |
| + | distinguishing feature of a slow sand filter is the presence of a thin layer, called | ||
| + | the schmutzdecke, which forms on the surface of the sand bed and includes a | ||
| + | a large variety of biologically active micro-organisms. | ||
| − | Water is introduced at the top and trickles down through the sand bed to the under- drains and goes to the storage tank. The impurities in the water are retained at the upper layers of the sand bed. In the process, the schmutzdecke consisting of bacteria and microscopic plants grow. The schmutzdecke removes the organic matter and most of the pathogenic micro-organisms in water which might be smaller than the pores of the sand. | + | Water is introduced at the top and trickles down through the sand bed to the |
| + | under-drains and goes to the storage tank. The impurities in the water are | ||
| + | retained at the upper layers of the sand bed. In the process, the schmutzdecke | ||
| + | consisting of bacteria and microscopic plants which grow. The schmutzdecke | ||
| + | removes the organic matter and most of the pathogenic micro-organisms in | ||
| + | water which might be smaller than the pores of the sand. | ||
| − | + | [[Image:Figure7.23.JPG|642px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | + | '''(a)Elements of a Slow Sand filter'''<br> | |
Figure 3.43(a) presents, in diagrammatic form the various elements that go to make up a Slow Sand Filter. Essentially the SSF elements consist of: | Figure 3.43(a) presents, in diagrammatic form the various elements that go to make up a Slow Sand Filter. Essentially the SSF elements consist of: | ||
(i)a supernatant (raw) water reservoir, the principal function of which is to maintain a constant head of water above the filter medium, this head providing the pressure that carries the water through the filter;<br> | (i)a supernatant (raw) water reservoir, the principal function of which is to maintain a constant head of water above the filter medium, this head providing the pressure that carries the water through the filter;<br> | ||
| Line 230: | Line 229: | ||
(vi)It is a very simple and effective technique for purifying surface water. It will remove practically all of the turbidity from the water as well as most of the pathogens without the addition of chemicals. Slow sand filters can frequently be constructed largely from locally-available materials.<br> | (vi)It is a very simple and effective technique for purifying surface water. It will remove practically all of the turbidity from the water as well as most of the pathogens without the addition of chemicals. Slow sand filters can frequently be constructed largely from locally-available materials.<br> | ||
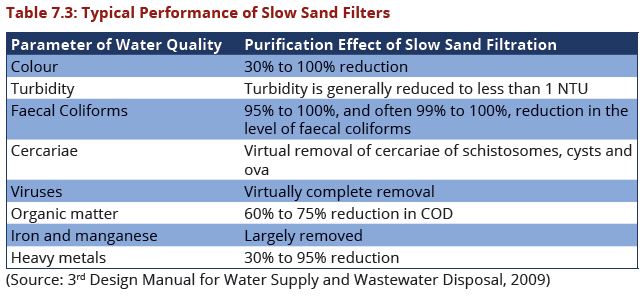
| − | In | + | In an SSF the water is purified by slow percolation through a bed of fine sand. |
| − | + | Pre-treatment is necessary with raw waters having average turbidity of 25 | |
| + | NTU or more, but should be considered also for less turbid raw waters 5 to 25 | ||
| + | NTU) to improve effluent quality and reduce the frequency of cleaning. The SSF | ||
| + | is also useful for treating groundwater containing solids in suspension, e.g. ferric | ||
| + | and manganese compounds converted by aeration from the soluble state of the | ||
| + | salts. | ||
| − | + | [[Image:Table7.3.JPG|643px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | '''(b)Design Considerations of Slow Sand Filter (SSF)'''<br> | |
* Raw water quality and necessity for pre—treatment and/or aeration; | * Raw water quality and necessity for pre—treatment and/or aeration; | ||
| Line 265: | Line 248: | ||
* The filter sand must be free from any clay or silt content and preferably of a well- rounded quartz material. Organic matter should be avoided. | * The filter sand must be free from any clay or silt content and preferably of a well- rounded quartz material. Organic matter should be avoided. | ||
| − | + | '''(c)Design Criteria;'''<br> | |
* When choosing the filter sand, the grain size distribution should meet the effective size 0.15 to 0.35 mm and the coefficient of uniformity should be less than 3 , | * When choosing the filter sand, the grain size distribution should meet the effective size 0.15 to 0.35 mm and the coefficient of uniformity should be less than 3 , | ||
* In order to calculate for the total area of filter beds, a working rate of 0.1 - 0.2, m3/m2/hr is recommended. When one filter is not operational, the working rate of the remaining filter should not exceed 0.2 m3/m2/h. | * In order to calculate for the total area of filter beds, a working rate of 0.1 - 0.2, m3/m2/hr is recommended. When one filter is not operational, the working rate of the remaining filter should not exceed 0.2 m3/m2/h. | ||
* The turbidity in the incoming water should not exceed an average of 5 - 10 NTU. In cases of higher turbidity, preliminary treatment such as roughing filters is necessary | * The turbidity in the incoming water should not exceed an average of 5 - 10 NTU. In cases of higher turbidity, preliminary treatment such as roughing filters is necessary | ||
* The inlet structure should be designed in such a way that the raw water is equally distributed over the filter bed area. | * The inlet structure should be designed in such a way that the raw water is equally distributed over the filter bed area. | ||
| − | * To achieve this, the inlet velocity should be around 0.1 m/s and the width of the inlet structure should be at least (0.05 x Q) | + | * To achieve this, the inlet velocity should be around 0.1 m/s and the width of the inlet structure should be at least (0.05 x Q) meters, where Q is the design flow in m3/h. |
* The minimum size of a filter unit should be 15 to 20 m2; | * The minimum size of a filter unit should be 15 to 20 m2; | ||
* The height of the supernatant water should be 1 to 1.5 m, | * The height of the supernatant water should be 1 to 1.5 m, | ||
| Line 276: | Line 259: | ||
* Calculate filter surface area = flow capacity (m3) / rate of filtration | * Calculate filter surface area = flow capacity (m3) / rate of filtration | ||
| − | + | '''(d)Main Water Under-drain''' <br> | |
* Calculate Diameter of the under drain = (22 × d2) / (28 × r) | * Calculate Diameter of the under drain = (22 × d2) / (28 × r) | ||
* Area of holes or slots to be 1.5% of area of the filter | * Area of holes or slots to be 1.5% of area of the filter | ||
| Line 286: | Line 269: | ||
* Area (A) per filter bed = 10 - 100 m2 | * Area (A) per filter bed = 10 - 100 m2 | ||
| − | + | '''Rapid Gravity Sand Filtration'''<br> | |
| − | This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media | + | This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media that actually removes the particles from the water. The filter media is routinely cleaned by means of a backwashing process. |
| − | Rapid Sand Filtration (RSF) is a technique commonly used for treating large quantities of drinking water. It is a relatively sophisticated process usually requiring power- operated pumps for backwashing or cleaning the filter bed, and some designs require flow control of the filter outlet. A continuously operating filter will usually require backwashing about every two days or so when raw water is of relatively low turbidity and at least daily during periods of high turbidity. Because of the higher filtration rates, the area | + | Rapid Sand Filtration (RSF) is a technique commonly used for treating large quantities of drinking water. It is a relatively sophisticated process usually requiring power- operated pumps for backwashing or cleaning the filter bed, and some designs require flow control of the filter outlet. A continuously operating filter will usually require backwashing about every two days or so when raw water is of relatively low turbidity and at least daily during periods of high turbidity. Because of the higher filtration rates, the area required for a rapid gravity filtration plant is about 20% of that required for slow sand filters. |
Surface loading should be between 4 and 7 m3/h.m2, and the filter structure should be designed with a minimum height between the top of the filter media and the bottom of the wash water channel of at least 30% of the height of the filter media as this expands during backwashing. It may be necessary to include for air-scour as well as backwashing, or for the two combined in a single operation. | Surface loading should be between 4 and 7 m3/h.m2, and the filter structure should be designed with a minimum height between the top of the filter media and the bottom of the wash water channel of at least 30% of the height of the filter media as this expands during backwashing. It may be necessary to include for air-scour as well as backwashing, or for the two combined in a single operation. | ||
| Line 312: | Line 295: | ||
In order to achieve proper washing of the filter a storage volume sufficient for continuous washing for an 8 to 10 minute period should be available. | In order to achieve proper washing of the filter a storage volume sufficient for continuous washing for an 8 to 10 minute period should be available. | ||
| − | + | '''(b)Design Steps'''<br> | |
The following design steps should be followed; | The following design steps should be followed; | ||
| Line 323: | Line 306: | ||
* Select flow capacity and flow velocity | * Select flow capacity and flow velocity | ||
* Calculate area required = (low capacity)/ (low velocity) (m2) | * Calculate area required = (low capacity)/ (low velocity) (m2) | ||
| − | * Calculate diameter of the | + | * Calculate diameter of the underdrain, d= Square Root of (4A/Π) |
* Area of holes = 0.2 - 0.4 % of the area of filter | * Area of holes = 0.2 - 0.4 % of the area of filter | ||
* Use five layers of sand particles in the filter as indicated above: | * Use five layers of sand particles in the filter as indicated above: | ||
| Line 329: | Line 312: | ||
* Sand depth 1 – 1.2 m | * Sand depth 1 – 1.2 m | ||
| − | + | '''(c)Filter Backwashing''' <br> | |
A major cause of poor performance by rapid gravity filters is a result of either inadequate or excessive backwashing rates. Backwashing is sometimes carried out by water alone but more often by air and water usually applied one after the other by reverse flow to the filter bed. The first operation however is to allow the filter to drain down until the water lies a few centimetres above the top of the bed. Air is then introduced through the collector system at a rate of about 6.5 to 7.5 mm/s. | A major cause of poor performance by rapid gravity filters is a result of either inadequate or excessive backwashing rates. Backwashing is sometimes carried out by water alone but more often by air and water usually applied one after the other by reverse flow to the filter bed. The first operation however is to allow the filter to drain down until the water lies a few centimetres above the top of the bed. Air is then introduced through the collector system at a rate of about 6.5 to 7.5 mm/s. | ||
Where air and water is applied separately, air scour normally lasts about 3 – 4 minutes and the water wash about 4 – 6 minutes. Where applied concurrently, air is first introduced and after about 1.5 – 2 minutes when it is fully established water is introduced and the combined backwash last for about 6 – 8 minutes. Air is stopped first and the water run for several more minutes to rinse the bed. Generally, total water consumption per wash amounts to about 2.5 bed volumes, but should normally not exceed 2% of the treated water output in well run plants. | Where air and water is applied separately, air scour normally lasts about 3 – 4 minutes and the water wash about 4 – 6 minutes. Where applied concurrently, air is first introduced and after about 1.5 – 2 minutes when it is fully established water is introduced and the combined backwash last for about 6 – 8 minutes. Air is stopped first and the water run for several more minutes to rinse the bed. Generally, total water consumption per wash amounts to about 2.5 bed volumes, but should normally not exceed 2% of the treated water output in well run plants. | ||
| − | '''Comparison between Slow Sand Filters and Rapid Sand Filters''' | + | '''Comparison between Slow Sand Filters and Rapid Sand Filters'''<br> |
a)Base material: In SSF it varies from 3 to 65 mm in size and 30 to 75 cm in depth while in RSF it varies from 3 to 40 mm in size and its depth is slightly more, i.e. about 60 to 90 cm.<br> | a)Base material: In SSF it varies from 3 to 65 mm in size and 30 to 75 cm in depth while in RSF it varies from 3 to 40 mm in size and its depth is slightly more, i.e. about 60 to 90 cm.<br> | ||
b)Filter sand: In SSF the effective size ranges between 0.2 to 0.4 mm and uniformity coefficient between 1.8 to 2.5 or 3.0. In RSF the effective size ranges between 0.35 to 0.55 and uniformity coefficient between 1.2 to 1.8.<br> | b)Filter sand: In SSF the effective size ranges between 0.2 to 0.4 mm and uniformity coefficient between 1.8 to 2.5 or 3.0. In RSF the effective size ranges between 0.35 to 0.55 and uniformity coefficient between 1.2 to 1.8.<br> | ||
c)Rate of filtration: In SSF it is small, such as 100 to 200 L/h/sq.m. of filter area while in RSF it is large, such as 3000 to 6000 L/h/sq.m. of filter area.<br> | c)Rate of filtration: In SSF it is small, such as 100 to 200 L/h/sq.m. of filter area while in RSF it is large, such as 3000 to 6000 L/h/sq.m. of filter area.<br> | ||
d)Flexibility: SSF are not flexible for meeting variation in demand whereas RSF are quite flexible for meeting reasonable variations in demand.<br> | d)Flexibility: SSF are not flexible for meeting variation in demand whereas RSF are quite flexible for meeting reasonable variations in demand.<br> | ||
| − | e)Post treatment required: Almost pure water is obtained from SSF. However, water may be disinfected slightly to make it completely safe. Disinfection is a must after RSF.<br> | + | e) Post-treatment required: Almost pure water is obtained from SSF. However, water may be disinfected slightly to make it completely safe. Disinfection is a must after RSF.<br> |
f)Method of cleaning: Scrapping and removing of the top 1.5 to 3 cm thick layer is done to clean SSF. To clean RSF, sand is agitated and backwashed with or without compressed air.<br> | f)Method of cleaning: Scrapping and removing of the top 1.5 to 3 cm thick layer is done to clean SSF. To clean RSF, sand is agitated and backwashed with or without compressed air.<br> | ||
g)Loss of head: In case of SSF approx. 10 cm is the initial loss, and 0.8 to 1.2m is the final limit when cleaning is required. For RSF 0.3m is the initial loss, and 2.5 to 3.5m is the final limit when cleaning is required. | g)Loss of head: In case of SSF approx. 10 cm is the initial loss, and 0.8 to 1.2m is the final limit when cleaning is required. For RSF 0.3m is the initial loss, and 2.5 to 3.5m is the final limit when cleaning is required. | ||
| − | + | '''Other Types of Filters'''<br> | |
| − | + | '''(a)Pressure Filters'''<br> | |
| − | Pressure filters are circular | + | Pressure filters are circular pressurized vessels containing the filter media and usually designed for vertical flow. They work on the same principle as rapid gravity filters differing in that the filter medium is enclosed in a steel vessel and the water is forced through it under pressure. |
| − | + | '''(b)Upward Flow Filters'''<br> | |
Upward flow filters are theoretically more efficient than gravity filters where the water to be filtered flows upwards through the naturally desegregated, progressively finer and finer media so that coarser particles are trapped first in the coarser bottom layers. This tends to extend the period between backwashing. Several sand grades, getting progressively finer upwards have also been used in which case some restraining means such as a grid is required often located about 0.1 m below the surface where upward backwashing is used. | Upward flow filters are theoretically more efficient than gravity filters where the water to be filtered flows upwards through the naturally desegregated, progressively finer and finer media so that coarser particles are trapped first in the coarser bottom layers. This tends to extend the period between backwashing. Several sand grades, getting progressively finer upwards have also been used in which case some restraining means such as a grid is required often located about 0.1 m below the surface where upward backwashing is used. | ||
| − | The major reason why RSF is a preferred option of water filtration to SSF is rate of filtration where RSF is higher per filter area flexibility, where SSF | + | The major reason why RSF is a preferred option of water filtration to SSF is the rate of filtration where RSF is higher per filter area flexibility, where SSF is not flexible for meeting variation in demand, method of cleaning wherein the case of RSF, no sand is lost. Also, RSF requires minimal land requirements (Source: Ugandan Water Supply Design Manual (2013). |
| − | + | '''(c)Roughing Filters'''<br> | |
Roughing filters have their place as a form of primary treatment, especially for turbid or highly changeable river water. This technique of primary treatment has been greatly under-utilised in Tanzania in the past. Although much research has been undertaken since late 1970s (Mbwette & Wegelin, 1984). It is used primarily to remove solids from high turbidity source waters prior to treatment with such unit operations as slow sand filters. | Roughing filters have their place as a form of primary treatment, especially for turbid or highly changeable river water. This technique of primary treatment has been greatly under-utilised in Tanzania in the past. Although much research has been undertaken since late 1970s (Mbwette & Wegelin, 1984). It is used primarily to remove solids from high turbidity source waters prior to treatment with such unit operations as slow sand filters. | ||
| − | In a typical roughing filter, there are series of tanks which are filled with progressively smaller diameter media in the direction of the flow which can either be horizontal or vertical. With respect to the vertical flow direction, up-flow or down-flow roughing filters can be designed. The media in the tank which may include gravel, rice husks or any other suitable local material, plays an important role | + | In a typical roughing filter, there are series of tanks which are filled with progressively smaller diameter media in the direction of the flow which can either be horizontal or vertical. With respect to the vertical flow direction, up-flow or down-flow roughing filters can be designed. The media in the tank which may include gravel, rice husks or any other suitable local material, plays an important role in reducing the vertical settling distance of the particles to a distance of a few millimeters |
| − | + | '''(i)Advantages of Roughing filters'''<br> | |
* Can considerably reduce the number of pathogens in the water, as well as the amount of iron and manganese. | * Can considerably reduce the number of pathogens in the water, as well as the amount of iron and manganese. | ||
* Can be considered a major pre-treatment process for turbid surface water since they efficiently separate fine solid particles over prolonged periods. | * Can be considered a major pre-treatment process for turbid surface water since they efficiently separate fine solid particles over prolonged periods. | ||
* Long filters (10 m) at low filtration rates (0.5 m/h) are capable of reducing high suspended solids concentrations (1000 mg/l TSS down to less than 3 mg/l). | * Long filters (10 m) at low filtration rates (0.5 m/h) are capable of reducing high suspended solids concentrations (1000 mg/l TSS down to less than 3 mg/l). | ||
| − | * Capable of reducing peak turbidities by 80 to 90 percent and | + | * Capable of reducing peak turbidities by 80 to 90 percent and fecal coliforms by 77 to 89 percent. |
* They are placed at the treatment plant site and operated in combination with other pre-treatment units such as dynamic filters or sedimentation tanks. | * They are placed at the treatment plant site and operated in combination with other pre-treatment units such as dynamic filters or sedimentation tanks. | ||
| − | NB: For detailed information, designers are recommended to download the comprehensive SANDTEC design report entitled | + | '''NB:''' For detailed information, designers are recommended to download the |
| + | comprehensive SANDTEC design report entitled ‘''Surface Water Treatment by | ||
| + | Roughing Filters, A Design, Construction and Operation Manual’'' available at http// | ||
| + | sandec.ch/WaterTreatment/Documents/Surface%20Water%20Treatment.pdf. | ||
| + | |||
| + | Features of Roughing Filters (RF). | ||
The main part of the filter is the section containing the filter material. However, a Roughing filter comprises the following six elements:- | The main part of the filter is the section containing the filter material. However, a Roughing filter comprises the following six elements:- | ||
| Line 375: | Line 363: | ||
# Drainage System | # Drainage System | ||
| − | + | '''(ii)Parameters for Design of Roughing Filter'''<br> | |
# Filter media size | # Filter media size | ||
# Filtration Rates | # Filtration Rates | ||
| Line 381: | Line 369: | ||
# Filter media materials | # Filter media materials | ||
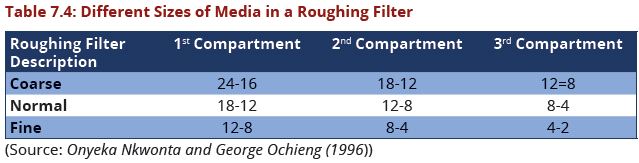
| − | + | '''(iii)Filter Media Size'''<br> | |
*The size of filter media decreases successively in the direction of water flow and ideally the uniformity of filter media fractions is maximized to increase filter pore space (storage capacity) and aid in filter cleaning (Boller, 1993). | *The size of filter media decreases successively in the direction of water flow and ideally the uniformity of filter media fractions is maximized to increase filter pore space (storage capacity) and aid in filter cleaning (Boller, 1993). | ||
*The use of multiple grades of filter media in a roughing filter promotes the penetration of particles throughout the filter bed and takes advantage of the large storage capacities offered by larger media and high removal efficiencies offered by small media | *The use of multiple grades of filter media in a roughing filter promotes the penetration of particles throughout the filter bed and takes advantage of the large storage capacities offered by larger media and high removal efficiencies offered by small media | ||
| Line 388: | Line 376: | ||
*Common grades of media used in roughing filters are provided in the Table 7.4 | *Common grades of media used in roughing filters are provided in the Table 7.4 | ||
| − | + | [[Image:Table7.4.JPG|639px|link=Chapter_Seven:_Water_Treatment]] <br> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | '''(iv)Filtration Rates'''<br> | |
* Filtration rate has a significant influence on the treatment removal. | * Filtration rate has a significant influence on the treatment removal. | ||
* Good removal in roughing filters are best achieved with low filtration rate (Boller, 1993), because low filtration rates are critical to retain particles that are gravitationally deposited to the surface of the media. | * Good removal in roughing filters are best achieved with low filtration rate (Boller, 1993), because low filtration rates are critical to retain particles that are gravitationally deposited to the surface of the media. | ||
| − | * While used as pretreatments for iron and manganese removal, it can operate at filtration rates of 1.5 - 3 m/h (Hatva, 1988). It is reported that horizontal flow roughing filter is capable of removing metals like iron, manganese, turbidity and color at a filtration rate of 1.8 m/h (Dastanaie, 2007) | + | * While used as pretreatments for iron and manganese removal, it can operate at filtration rates of 1.5 - 3 m/h (Hatva, 1988). It is reported that horizontal flow roughing filter is capable of removing metals like iron, manganese, turbidity, and color at a filtration rate of 1.8 m/h (Dastanaie, 2007) |
| − | * At increased filtration rates (2 m/h), coarse particles penetrate deeper into the bed and they cause decrease in filter efficiency (Wegelin et al. (1986). Whereas at 1 m/h there is good distribution of solids loading throughout the bed. Hendricks (1991) also suggested that normal filtration rate of horizontal roughing filters is between 0.3 and 1.5 m/h. | + | * At increased filtration rates (2 m/h), coarse particles penetrate deeper into the bed and they cause a decrease in filter efficiency (Wegelin et al. (1986). Whereas at 1 m/h there is good distribution of solids loading throughout the bed. Hendricks (1991) also suggested that normal filtration rate of horizontal roughing filters is between 0.3 and 1.5 m/h. |
| − | + | '''(v)Filter Media Length'''<br> | |
* Improved cumulative removal efficiencies are typically correlated to longer filter lengths (Collins, 1994; Wegelin, 1986). | * Improved cumulative removal efficiencies are typically correlated to longer filter lengths (Collins, 1994; Wegelin, 1986). | ||
| − | * Incremental removal efficiencies | + | * Incremental removal efficiencies decrease with increasing filter length due to the preferential removal of larger particles early in the filter (Wegelin, 1996). |
| − | * The rate of decline is dependent on filter design variables and the size and nature of particles in suspension. The | + | * The rate of decline is dependent on filter design variables and the size and nature of particles in suspension. The use of different media sizes may allow for treatment targets to be met by a shorter filter with multiple media sizes compared to with long filter packed with one media size. |
| − | + | '''(vi)Filter Media Materials'''<br> | |
The following material could therefore be used as filter media: | The following material could therefore be used as filter media: | ||
a)Gravel from a river bed or from the ground.<br> | a)Gravel from a river bed or from the ground.<br> | ||
| Line 418: | Line 395: | ||
c)Broken burnt clay bricks.<br> | c)Broken burnt clay bricks.<br> | ||
d)Plastic material either as chips or modules (e.g. used for trickling filters) may be used if the material is locally available.<br> | d)Plastic material either as chips or modules (e.g. used for trickling filters) may be used if the material is locally available.<br> | ||
| − | e)Burnt charcoal, although there is a risk of disintegration | + | e)Burnt charcoal, although there is a risk of disintegration when cleaning the filter material, should only be considered in special cases (e.g. for removal of dissolved organic matter).<br> |
| − | f)Coconut | + | f)Coconut fiber, however, due to the risk of flavoring the water during long filter operation, should be used with care.<br> |
| − | g)Broken burnt bricks and improved agricultural | + | g)Broken burnt bricks and improved agricultural waste (e.g. charcoal, maize cobs), can also be effectively used as pretreatment media (Ochieng (2006) and therefore could serve as alternatives where natural gravel is not readily available. |
| − | + | '''(vii)Types of Roughing Filters'''<br> | |
| − | There are many types of roughing filters with different flow directions and with different types of filter medium (e.g. sand, gravel, coconut husk | + | There are many types of roughing filters with different flow directions and with different types of filter medium (e.g. sand, gravel, coconut husk fiber). However, the common types are: |
i.Horizontal Flow Filters (HRF)<br> | i.Horizontal Flow Filters (HRF)<br> | ||
ii.Vertical (VRF)<br> | ii.Vertical (VRF)<br> | ||
iii.Dynamic (DRF) | iii.Dynamic (DRF) | ||
| − | + | (d) Bank Filtration (BF) | |
Bank filtration (BF) is the infiltration of surface water, mostly from a river system into a groundwater system induced by water abstraction close to the surface water (e.g. river bank). This water abstraction is commonly done by operating wells. | Bank filtration (BF) is the infiltration of surface water, mostly from a river system into a groundwater system induced by water abstraction close to the surface water (e.g. river bank). This water abstraction is commonly done by operating wells. | ||
| − | As the water flows through the soil, it is filtered and hence its quality is improved. In the context of developing or newly | + | As the water flows through the soil, it is filtered and hence its quality is improved. In the context of developing or newly industrialized countries, bank filtration may contribute to a more sustainable water cycle by recharging stressed groundwater bodies with filtered surface water. (Sharma & Amy 2009; Huelshoff et al.) |
Bank filtration is a water treatment technology that consists of extracting water from rivers by pumping wells located in the adjacent alluvial aquifer. During the underground passage, a series of physical, chemical, and biological processes take place, improving the quality of the surface water, substituting or reducing conventional drinking water treatment. | Bank filtration is a water treatment technology that consists of extracting water from rivers by pumping wells located in the adjacent alluvial aquifer. During the underground passage, a series of physical, chemical, and biological processes take place, improving the quality of the surface water, substituting or reducing conventional drinking water treatment. | ||
| Line 442: | Line 419: | ||
One limitation on the efficiency of BF is the clogging of the bed and the banks of the river, which decreases the hydraulic conductivity in the hyporheic zone. This clogging can be caused by infiltration of the fine sediments, gas entrapment, bio-film formation related to microbiological activity, or the precipitation and co-precipitation of inorganic compounds, being the first of these the most influential factor in clogging formation.<br> | One limitation on the efficiency of BF is the clogging of the bed and the banks of the river, which decreases the hydraulic conductivity in the hyporheic zone. This clogging can be caused by infiltration of the fine sediments, gas entrapment, bio-film formation related to microbiological activity, or the precipitation and co-precipitation of inorganic compounds, being the first of these the most influential factor in clogging formation.<br> | ||
| − | '''Siting and Design Parameters''' | + | '''Siting and Design Parameters'''<br> |
# The most important parameters for success during BF are the flow path length, the thickness of the aquifer, and the infiltration area in the river (Grischek et al. (2002). | # The most important parameters for success during BF are the flow path length, the thickness of the aquifer, and the infiltration area in the river (Grischek et al. (2002). | ||
# The siting and design of a BF system depend on hydrogeological, technical, economical, regulatory, and land-use factors. | # The siting and design of a BF system depend on hydrogeological, technical, economical, regulatory, and land-use factors. | ||
| Line 451: | Line 428: | ||
==== Floatation ==== | ==== Floatation ==== | ||
| − | Floatation may be defined as the transfer of a suspended phase from the bulk of a dispersion medium to the atmosphere/liquid interface by means of bubble attachment. There are three basic processes involved which are: bubble generation, bubble attachment and solids separation. | + | Floatation may be defined as the transfer of a suspended phase from the |
| + | bulk of a dispersion medium to the atmosphere/liquid interface by means of | ||
| + | bubble attachment. There are three basic processes involved which are: bubble | ||
| + | generation, bubble attachment and solids separation. | ||
| − | Floatation is described as a gravity separation process, in which gas bubbles attach to solid particles to cause the apparent density of the bubble-solid agglomerates to be less than that of the water thereby allowing the agglomerates to float to the surface. The different methods of producing the gas bubbles give rise to different types of Floatation processes which are Dissolved-air Floatation, Electrolytic Floatation and Dispersed-air Floatation. | + | Floatation is described as a gravity separation process, in which gas bubbles attach |
| + | to solid particles to cause the apparent density of the bubble-solid agglomerates | ||
| + | to be less than that of the water thereby allowing the agglomerates to float to the | ||
| + | surface. The different methods of producing the gas bubbles give rise to different | ||
| + | types of Floatation processes which are Dissolved-air Floatation, Electrolytic | ||
| + | Floatation and Dispersed-air Floatation. | ||
==== Dissolved-Air Floatation ==== | ==== Dissolved-Air Floatation ==== | ||
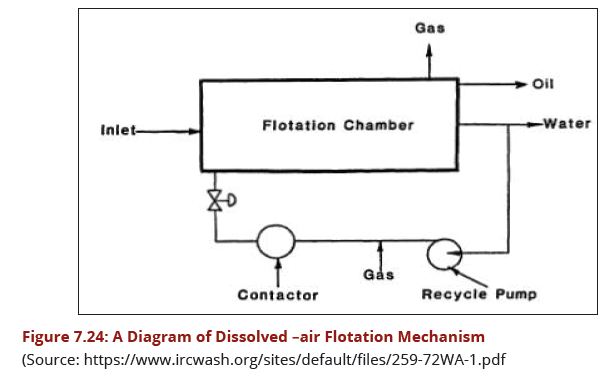
This is a relatively new solution for the clarification of surface and ground waters. The process as shown in Fig 7.24 is relatively simple and can be very effective. After flocculation, the produced floc attaches to micro-bubbles and rises to the water's surface. The floated solids are periodically evacuated either hydraulically or mechanically, depending on the sludge concentration required. | This is a relatively new solution for the clarification of surface and ground waters. The process as shown in Fig 7.24 is relatively simple and can be very effective. After flocculation, the produced floc attaches to micro-bubbles and rises to the water's surface. The floated solids are periodically evacuated either hydraulically or mechanically, depending on the sludge concentration required. | ||
| − | + | [[Image:Figure7.24.JPG|607px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
The dissolved air flotation process can be a good solution for the treatment of water with a high concentration of algae or other low density particles. The system is said to have a number of advantages including: | The dissolved air flotation process can be a good solution for the treatment of water with a high concentration of algae or other low density particles. The system is said to have a number of advantages including: | ||
| − | a.Reliable removal of algae, cryptosporidium and giardia cysts | + | a.Reliable removal of algae, cryptosporidium and giardia cysts<br> |
| − | b.Removal of colour and taste compounds | + | b.Removal of colour and taste compounds<br> |
| − | c.Removal of low-density solids | + | c.Removal of low-density solids<br> |
| − | d.No polymer required | + | d.No polymer required<br> |
| − | e.Concentrated sludge | + | e.Concentrated sludge<br> |
| − | f.Rapid start-up after shutdown | + | f.Rapid start-up after shutdown<br> |
| − | g.Few mechanical components | + | g.Few mechanical components<br> |
h.Low operating costs | h.Low operating costs | ||
| Line 487: | Line 467: | ||
Aeration is the process whereby water is brought into intimate contact with air. Aeration has a large number of uses in water treatment for the following purposes: | Aeration is the process whereby water is brought into intimate contact with air. Aeration has a large number of uses in water treatment for the following purposes: | ||
| − | i.Increasing dissolved oxygen content in the water; | + | i.Increasing dissolved oxygen content in the water;<br> |
| − | ii.Reducing tastes and odours caused by dissolved gases in the water, such as hydrogen sulphide, which are then released; and also to oxidise and remove organic matter; | + | ii.Reducing tastes and odours caused by dissolved gases in the water, such as hydrogen sulphide, which are then released; and also to oxidise and remove organic matter;<br> |
| − | iii.Decreasing carbon dioxide content of water and thereby reduce its corrosiveness and raise its pH value; | + | iii.Decreasing carbon dioxide content of water and thereby reduce its corrosiveness and raise its pH value;<br> |
iv.Oxidizing iron and manganese from their soluble states to their insoluble states and thereby cause them to precipitate so that they may be removed by clarification and filtration processes; | iv.Oxidizing iron and manganese from their soluble states to their insoluble states and thereby cause them to precipitate so that they may be removed by clarification and filtration processes; | ||
| − | v.Reduction of radon; and | + | v.Reduction of radon; and<br> |
vi.Removing certain volatile organic compounds. | vi.Removing certain volatile organic compounds. | ||
| Line 497: | Line 477: | ||
Chemicals removed or oxidized by Aeration | Chemicals removed or oxidized by Aeration | ||
| − | a)Ammonia | + | a)Ammonia<br> |
| − | b)Chlorine | + | b)Chlorine<br> |
| − | c)Carbon dioxide | + | c)Carbon dioxide<br> |
| − | d)Hydrogen sulphide | + | d)Hydrogen sulphide<br> |
| − | e)Methane | + | e)Methane<br> |
| − | f)Iron and Manganese | + | f)Iron and Manganese<br> |
g)Volatile organic chemicals, such as benzene (found in gasoline), or trichloroethylene, dichloroethylene, and perchloroethylene (used in dry- cleaning or industrial processes) | g)Volatile organic chemicals, such as benzene (found in gasoline), or trichloroethylene, dichloroethylene, and perchloroethylene (used in dry- cleaning or industrial processes) | ||
| − | Types of Aerators | + | '''Types of Aerators'''<br> |
Aerators fall into two categories: | Aerators fall into two categories: | ||
| Line 511: | Line 491: | ||
* Injection aerators | * Injection aerators | ||
| − | Falling Water Aerators | + | ==== Falling Water Aerators ==== |
| − | In the falling water aerators, water is dropped through air and in the second group air is introduced | + | In the falling water aerators, water is dropped through air and in the second group air is introduced into the water as small bubbles. Falling water aerators can be divided into: |
| − | + | * Spray Aerators | |
| − | + | * Multiple Tray Aerators | |
| − | + | * Cascade Aerators | |
==== Spray Aerators ==== | ==== Spray Aerators ==== | ||
Water is sprayed through nozzles upward into the atmosphere and broken up into either a mist or droplets. Water is directed vertically or at a slight inclination to the vertical. The installation consists of trays and fixed nozzles on a pipe grid with necessary outlet arrangement. | Water is sprayed through nozzles upward into the atmosphere and broken up into either a mist or droplets. Water is directed vertically or at a slight inclination to the vertical. The installation consists of trays and fixed nozzles on a pipe grid with necessary outlet arrangement. | ||
| − | + | '''(a)Design details of Spray Aerators'''<br> | |
* Nozzles usually have diameters varying from 10 to 40 mm, spaced along the pipe at intervals of 0.5 to 1m or more. Special (patented) types of corrosion resistant nozzles and sometimes plain openings in pipes, serving as orifices are used. | * Nozzles usually have diameters varying from 10 to 40 mm, spaced along the pipe at intervals of 0.5 to 1m or more. Special (patented) types of corrosion resistant nozzles and sometimes plain openings in pipes, serving as orifices are used. | ||
* The pressure required at the nozzle head is usually 7 m of water but in practice, varies from 2 – 9 m and the discharge rating per nozzle varies form 30 - 600 l/min | * The pressure required at the nozzle head is usually 7 m of water but in practice, varies from 2 – 9 m and the discharge rating per nozzle varies form 30 - 600 l/min | ||
| Line 526: | Line 506: | ||
* The ‗Dresden‘ type of nozzles gives very good results in removing CO2 and in adding O2 but is poor for radon removal. | * The ‗Dresden‘ type of nozzles gives very good results in removing CO2 and in adding O2 but is poor for radon removal. | ||
| − | + | '''(b)Multiple Tray Aerators'''<br> | |
These aerators consist of a series of trays with perforated bottoms. The trays are filled with coke, stone or ceramic balls, limestone, or other materials having a catalytic effect on iron removal. The primary purpose of the materials is providing additional surface contact area between the air and water. Through perforated pipes, the water is divided evenly over the upper tray, from which it trickles down, the droplets being dispersed and re-collected at each successive tray. Appendix B.5 (iii) illustrate the Multiple Tray Aerators. | These aerators consist of a series of trays with perforated bottoms. The trays are filled with coke, stone or ceramic balls, limestone, or other materials having a catalytic effect on iron removal. The primary purpose of the materials is providing additional surface contact area between the air and water. Through perforated pipes, the water is divided evenly over the upper tray, from which it trickles down, the droplets being dispersed and re-collected at each successive tray. Appendix B.5 (iii) illustrate the Multiple Tray Aerators. | ||
| − | + | '''(c)Design details of Multiple Tray Aerators'''<br> | |
* 3-5 trays are normally used at the intervals of 0.3 - 0.7 m which means that the head needed is 1.5 – 3 m. | * 3-5 trays are normally used at the intervals of 0.3 - 0.7 m which means that the head needed is 1.5 – 3 m. | ||
* The area required is 40 m2 per 1,000 m3/hr. These aerators have good CO2 removal and good 02 increases (3rd edition Design Manual, 2009). | * The area required is 40 m2 per 1,000 m3/hr. These aerators have good CO2 removal and good 02 increases (3rd edition Design Manual, 2009). | ||
| Line 538: | Line 518: | ||
* difficult cleaning and breeding places for worms | * difficult cleaning and breeding places for worms | ||
| − | + | '''(d)Cascade Aerators (Gravity Aerator)'''<br> | |
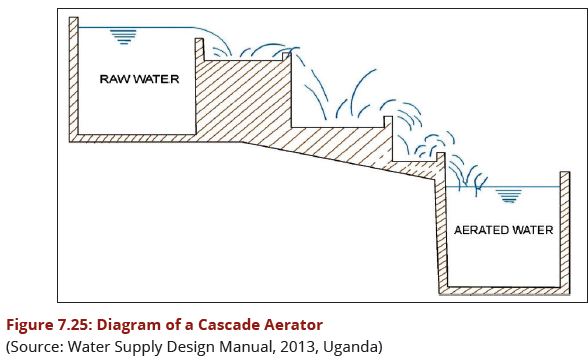
The Cascade Aerators are the simplest type of free-fall aerators and will take large quantities of water in a comparatively small area and at low head. They are simple to keep clean and can be made of robust durable material such as reinforced concrete and are best in the open air. Turbulence is secured by allowing the water to pass through a series of steps or baffles (3rd edition Design Manual, 2009). | The Cascade Aerators are the simplest type of free-fall aerators and will take large quantities of water in a comparatively small area and at low head. They are simple to keep clean and can be made of robust durable material such as reinforced concrete and are best in the open air. Turbulence is secured by allowing the water to pass through a series of steps or baffles (3rd edition Design Manual, 2009). | ||
| − | + | '''(e)Features of Cascade Aerator'''<br> | |
a)A cascade aerator consists of a flight of 4 - 6 steps, each about 300 mm high, to produce turbulence and thus enhance the aeration efficiency, obstacles are often sat at the edge of each step as illustrated in Figure 7.25. | a)A cascade aerator consists of a flight of 4 - 6 steps, each about 300 mm high, to produce turbulence and thus enhance the aeration efficiency, obstacles are often sat at the edge of each step as illustrated in Figure 7.25. | ||
b)The design capacity of a cascade aerator should be of the order of | b)The design capacity of a cascade aerator should be of the order of | ||
| Line 554: | Line 534: | ||
i)Where space permits, Cascade aerators are the preferred type of aerator. | i)Where space permits, Cascade aerators are the preferred type of aerator. | ||
| − | + | [[Image:Figure7.25.JPG|588px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | '''Design details of Cascade Aerators'''<br> | |
* Number of drops = 4-6 | * Number of drops = 4-6 | ||
* Height of drops = 30-60 cm | * Height of drops = 30-60 cm | ||
| − | * Overflow rate = 0.01 | + | * Overflow rate = 0.01 m<sup>3</sup>/s over meter width of step |
* Height of aerator = 2-3 m | * Height of aerator = 2-3 m | ||
| − | * Cascade area = 1.5-2.0 | + | * Cascade area = 1.5-2.0 m<sup>2</sup>/m<sup>3</sup>/min of flow |
| − | ===== | + | ===== Injection Aerators ===== |
| − | These aerators have a good efficiency in raising | + | These aerators have a good efficiency in raising O<sub>2</sub> content but poor CO<sub>2</sub> removal. They can be categorised as: |
| − | a)Bubble Aerators | + | a)Bubble Aerators<br> |
| − | b)Venturi Aerators | + | b)Venturi Aerators<br> |
| − | c)Brush Aerators | + | c)Brush Aerators<br> |
| − | d)Inka Aerators | + | d)Inka Aerators<br> |
| − | + | '''(a)Bubble Aerators'''<br> | |
In these aerators, air is blown to the bottom of the tank through porous filters. Bubble aerators are often applied to existing treatment plants where no spare hydraulic head is available | In these aerators, air is blown to the bottom of the tank through porous filters. Bubble aerators are often applied to existing treatment plants where no spare hydraulic head is available | ||
| − | ''' | + | '''Design details of Bubble Aerators'''<br> |
i.The depth of the tank is 3 - 4.5 m and the width about 2 times the depth. | i.The depth of the tank is 3 - 4.5 m and the width about 2 times the depth. | ||
| − | ii.Detention time in the tank is 10 - 20 minutes. | + | ii. Detention time in the tank is 10 - 20 minutes. |
| − | iii.Air required is 40 – 120 m2 per 1,000 m3/hr, being 40% to 80% of water capacity. | + | iii. Air required is 40 – 120 m2 per 1,000 m3/hr, being 40% to 80% of water capacity. |
| − | iv.The air bubbles should be as small as possible but the clogging of the filters interfere with that aspect. | + | iv. The air bubbles should be as small as possible but the clogging of the filters interfere with that aspect. |
| − | v.Good mixing in an aeration tank improves efficiency | + | v.Good mixing in an aeration tank improves efficiency. |
| − | '''(b)Venturi Aerators''' | + | '''(b)Venturi Aerators'''<br> |
| − | In venturi aerators air is not blown into the water but is drawn in by a venturi. The 02 improvements is good but CO2 removal is poor. | + | In venturi aerators, air is not blown into the water but is drawn in by a venturi. The 02 improvements is good but CO2 removal is poor. |
| − | '''(c)Brush Aerator''' | + | '''(c)Brush Aerator'''<br> |
This type of aerator consists of a revolving drum, diameter about 0.5 m, submerged about 0.4m of the diameter, and rotating about 100 rpm. However, this type is not commonly used for water treatment. | This type of aerator consists of a revolving drum, diameter about 0.5 m, submerged about 0.4m of the diameter, and rotating about 100 rpm. However, this type is not commonly used for water treatment. | ||
| − | '''(d)Inka Aerators''' | + | '''(d)Inka Aerators'''<br> |
| − | This aerator consists of a perforated stainless steel plate under which air is blown. Water flows over the plate. The air water ratio is very high. It can be as high as 100:1. This amount of air causes heavy turbulence and so O2 raising efficiency and CO2 removal are good. Energy consumption is rather high corresponding to a hydraulic head of 7m if air water ratio is 100. A disadvantage is the clogging of the perforated plate. | + | This aerator consists of a perforated stainless steel plate under which air is blown. Water flows over the plate. The air-water ratio is very high. It can be as high as 100:1. This amount of air causes heavy turbulence and so O2 raising efficiency and CO2 removal are good. Energy consumption is rather a high corresponding to a hydraulic head of 7m if air-water ratio is 100. A disadvantage is the clogging of the perforated plate. |
=== Secondary Treatment === | === Secondary Treatment === | ||
| Line 640: | Line 615: | ||
* Method of cleaning | * Method of cleaning | ||
| − | + | A detailed description of filtration design considerations, criteria and steps have been given in the primary treatment section. | |
| − | + | === Tertiary Treatment === | |
Tertiary treatment has considered the following unit operations | Tertiary treatment has considered the following unit operations | ||
* Disinfection, | * Disinfection, | ||
| Line 649: | Line 624: | ||
* Water conditioning | * Water conditioning | ||
| − | |||
The single most important requirement of drinking water is that it should be free from any micro-organisms that could transmit disease or illness to the consumer. Processes such as storage, sedimentation, coagulation and flocculation and rapid filtration reduce to varying degrees the bacterial content of water. However these processes cannot assure that the water they produce is bacteriologically safe, therefore disinfection is finally needed. Disinfection is carried out observing the following criteria: | The single most important requirement of drinking water is that it should be free from any micro-organisms that could transmit disease or illness to the consumer. Processes such as storage, sedimentation, coagulation and flocculation and rapid filtration reduce to varying degrees the bacterial content of water. However these processes cannot assure that the water they produce is bacteriologically safe, therefore disinfection is finally needed. Disinfection is carried out observing the following criteria: | ||
| Line 659: | Line 633: | ||
* The pH, acidity/alkalinity of the water | * The pH, acidity/alkalinity of the water | ||
| − | ===== Disinfection Methods ===== | + | ===== ''Disinfection Methods'' ===== |
| − | There are two | + | There are two principal methods for disinfecting water; one is physical and the other chemical. Further details about disinfection methods are given in Appendix H. |
| − | ===== Chlorinators ===== | + | ===== ''Chlorinators'' ===== |
A chlorinator is a device designed for feeding chlorine in to a water supply. Its functions are: | A chlorinator is a device designed for feeding chlorine in to a water supply. Its functions are: | ||
| Line 678: | Line 652: | ||
* A ―desiccator valve‖ or non-return valve containing concentrated sulphuric acid or calcium chloride through which the chlorine must pass to free it from moisture so that any corrosive action of the moist chlorine on the fitting is prevented. | * A ―desiccator valve‖ or non-return valve containing concentrated sulphuric acid or calcium chloride through which the chlorine must pass to free it from moisture so that any corrosive action of the moist chlorine on the fitting is prevented. | ||
| − | '''Design considerations for chlorinators''' | + | '''Design considerations for chlorinators'''<br> |
The following should be considered while design chlorinators; | The following should be considered while design chlorinators; | ||
* Access to storage and dosing rooms should separate and be from the open air and doors should always open outwards. | * Access to storage and dosing rooms should separate and be from the open air and doors should always open outwards. | ||
* External windows should be avoided where possible with artificial illumination being provided throughout. | * External windows should be avoided where possible with artificial illumination being provided throughout. | ||
* Both storage and dosing rooms should be provided with low level outlet venting fans that either come on automatically when the door is opened or are activated from outside the room so that any leakage is purged to the outside before entering such rooms. | * Both storage and dosing rooms should be provided with low level outlet venting fans that either come on automatically when the door is opened or are activated from outside the room so that any leakage is purged to the outside before entering such rooms. | ||
| − | * High level fresh air inlets should be provided, especially to the storage room. | + | * High-level fresh air inlets should be provided, especially to the storage room. |
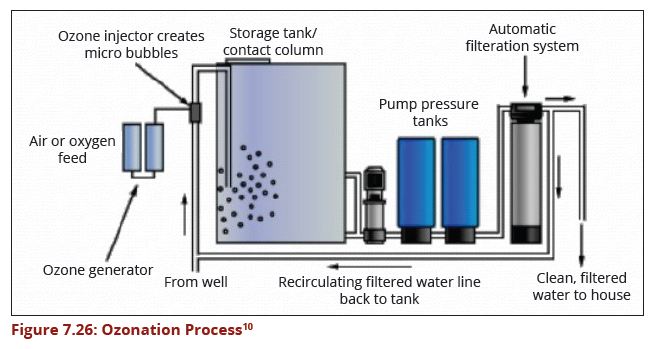
==== Ozonation ==== | ==== Ozonation ==== | ||
Ozone has been proved to be one of the most effective disinfectants and is widely used to inactivate pathogens in drinking water (Xu, 2002). Transferred ozone dose is the critical parameter for the design of wastewater disinfection by ozonation. The process should have an efficient filtration step to meet stringent standards. A properly designed ozonation process is able to deactivate viruses and bacteria that may be contained in wastewater. | Ozone has been proved to be one of the most effective disinfectants and is widely used to inactivate pathogens in drinking water (Xu, 2002). Transferred ozone dose is the critical parameter for the design of wastewater disinfection by ozonation. The process should have an efficient filtration step to meet stringent standards. A properly designed ozonation process is able to deactivate viruses and bacteria that may be contained in wastewater. | ||
| − | + | [[Image:Figure7.26.JPG|649px|link=Chapter_Seven:_Water_Treatment]] | |
| − | |||
| − | |||
| − | |||
| − | + | === Water softening === | |
| − | Softening is the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The hardness or soap consuming power of water is due to presence of bicarbonates, carbonates, | + | Softening is the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The hardness or soap-consuming power of water is due to the presence of bicarbonates, carbonates, sulfates, chlorides, and nitrates of calcium and magnesium. The dissolved compounds have the following negative effects: |
(i)Soap destroying or increased soap consumption in laundries,<br> | (i)Soap destroying or increased soap consumption in laundries,<br> | ||
| Line 701: | Line 672: | ||
(iv)Serious difficulties and detrimental effects in the manufacturing processes, e.g. textile finishing, dyeing, canning, paper making, ice manufacturing, tanning etc. | (iv)Serious difficulties and detrimental effects in the manufacturing processes, e.g. textile finishing, dyeing, canning, paper making, ice manufacturing, tanning etc. | ||
| − | When water is hard, it can clog pipes and soap will dissolve in it less easily. In industrial scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems. Hard water leads to the build- up of | + | When water is hard, it can clog pipes and soap will dissolve in it less easily. In industrial-scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems. Hard water leads to the build-up of limescale, which can foul plumbing, and promote galvanic corrosion. Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. It is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Water softening is usually achieved using lime softening or ion-exchange resins but is increasingly being accomplished using Nanofiltration or reverse osmosis membranes |
Chemicals used for softening include calcium hydroxide (slaked lime) and sodium. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Detailed information about hardness is provided in Appendix I | Chemicals used for softening include calcium hydroxide (slaked lime) and sodium. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Detailed information about hardness is provided in Appendix I | ||
| Line 708: | Line 679: | ||
The most common means for removing water hardness (calcium and/or magnesium) and hence achieve softening are: | The most common means for removing water hardness (calcium and/or magnesium) and hence achieve softening are: | ||
| − | + | * Chemical precipitation; | |
| − | + | * Ion-exchange resin; and | |
| − | + | * Reverse Osmosis (RO) | |
| + | |||
| + | '''(a) Softening By Chemical Precipitation'''<br> | ||
| + | Chemical precipitation is among the most common methods used to soften | ||
| + | water. Chemicals used are lime (calcium hydroxide, Ca(OH)2) and soda ash | ||
| + | (sodium carbonate, Na2CO3). Softening by chemical precipitation is accomplished | ||
| + | by adding lime or lime and soda ash. Softening with these chemicals is used | ||
| + | particularly for water with high initial hardness greater than 500 mg/l and | ||
| + | suitable for water containing turbidity, colour, and iron salts because these have | ||
| + | a tendency to inactivate the ion exchange bed, by a coating on the granules. | ||
| + | Lime-soda softening cannot, however, reduce the hardness to values less than | ||
| + | 40 mg/l and this should not be attempted. | ||
| + | |||
| + | Ion-exchange softening can produce zero-hardness water but such water should | ||
| + | always be blended with water to leave a residual hardness of not less than 70 | ||
| + | mg/l because apart from the risk of cardiovascular problems, very soft drinking | ||
| + | water may be corrosive and result in feelings of sickness. | ||
| + | |||
| + | '''(i) Lime Treatment'''<br> | ||
| + | Lime softening is the process in which lime is added to hard water to make it | ||
| + | softer. It has several advantages over the ion-exchange method but requires fulltime, | ||
| + | trained personnel to run the equipment. Addition of lime to hard water only | ||
| + | removes the carbonate hardness. Insoluble carbonates of calcium and magnesium | ||
| + | are precipitated out and removed in sedimentation tanks. (An overdose of lime | ||
| + | is usually used and the excess lime is neutralized by re-carbonation before | ||
| + | filtration). This treatment is good when the bulk of the hardness is due to calcium | ||
| + | and magnesium is insignificant. When the water contains more than 40 mg/l of | ||
| + | magnesium warranting its removal, excess lime treatment must be done. | ||
| + | |||
| + | '''(ii) Lime – Soda Treatment'''<br> | ||
| + | Lime is used to remove chemicals that cause carbonate hardness, while Sodaash | ||
| + | is used to remove chemicals that cause non-carbonate hardness. In lime | ||
| + | treatment only the carbonate hardness is removed but by addition of soda, the | ||
| + | non-carbonate hardness is also removed, thus the removal of both carbonate | ||
| + | as well as non-carbonate hardness is possible in the lime-soda process. This | ||
| + | happens because in lime-soda ash softening process Ca2+ is removed from water | ||
| + | in the form of calcium carbonate, CaCO<sub>3</sub> (s) and Mg<sub>2+</sub> is removed in the form | ||
| + | of magnesium hydroxide, Mg(OH)<sub>2</sub> (s). These precipitates are then removed by | ||
| + | conventional processes of coagulation/flocculation, sedimentation, and filtration. | ||
| + | Because precipitates are very slightly soluble, some hardness remains in the | ||
| + | water usually about 50 to 85 mg/l (as CaCO3). This hardness level is desirable to | ||
| + | prevent corrosion problems associated with water being too soft and having little | ||
| + | or no hardness. Precipitation of these salts is affected by the available Carbonate | ||
| + | species and pH of the system | ||
| + | * For calculating the theoretical amount of lime and soda required for softening, an analysis of the following constituents in the water is necessary: | ||
| + | * free carbon dioxide dissolved in the water bicarbonate (total alkalinity) | ||
| + | * total hardness | ||
| + | * total magnesium | ||
| + | |||
| + | Chemical requirement (mg/l) are computed by the sum of the following factors: | ||
| + | * Lime requirements as Ca(OH)<sub>2</sub> (100% purity) | ||
| + | – 56/44 of concentration of CO<sub>2</sub> (mg/l as CO<sub>2</sub>) | ||
| + | – 56/24 of concentration of Mg (mg/l as Mg) | ||
| + | – 56/100 of concentration of alkalinity (mg/l as CaCO<sub>3</sub>) | ||
| + | Additional lime required for raising the pH to the range of 10 to 10.5 for | ||
| + | precipitation of Mg(OH)<sub>2</sub> is about 30 - 50 mg/l as CaO (Quick lime). | ||
| + | |||
| + | Soda requirements as Na<sup>2</sup>CO<sub>3</sub> | ||
| + | – 106/100 of difference between total hardness and bicarbonate alkalinity | ||
| + | both expressed as CaCO<sub>3</sub>. | ||
| + | – For neutralizing excess lime at 30 mg/l, additional soda required is | ||
| + | (30/56) x 106 mg/l as Na<sup>2</sup>CO<sub>3</sub>. | ||
| + | Plant conditions like temperature; time of detention and agitation influence the | ||
| + | completeness of reactions and dosage of chemicals may have to be increased to | ||
| + | provide for the inadequacies. | ||
| + | |||
| + | Alternatively, caustic soda can be used instead of lime. Liquid caustic soda should | ||
| + | be used since it can be handled and fed easily. The amount calcium carbonate | ||
| + | sludge formed in this case is theoretically half that formed by use of lime. | ||
| + | However, using caustic soda is costlier than soda ash which is more expensive | ||
| + | than lime. | ||
| + | |||
| + | '''(iii) Excess Lime Treatment'''<br> | ||
| + | When water contains more than 40 mg/l of magnesium, excess lime treatment | ||
| + | has to be done since magnesium has to be removed as magnesium hydroxide | ||
| + | whose solubility decreases with increasing pH values. The water treated thus is | ||
| + | highly caustic and must be neutralised after precipitation either by re-carbonation | ||
| + | or by split treatment. In split treatment, the total flow is divided into two parts, | ||
| + | one part being treated with excess lime and the settled effluent then mixed with | ||
| + | un-softened water. The final residual hardness in the water will depend on the | ||
| + | percentage flow by-passed and the levels of or hardness in both the portions | ||
| + | (treated and by passed). | ||
| + | |||
| + | '''(iv) Hot Lime-Soda Treatment'''<br> | ||
| + | This process is used for boiler feed water treatment. It is similar to the cold | ||
| + | process already discussed except that the raw water is heated to about 95° - | ||
| + | 100°C before being taken’ to the reaction tank. Reactions take place rapidly due | ||
| + | to decreased viscosity hastening the settling of the precipitates. A greater degree | ||
| + | of softening is accomplished than that in the conventional cold processes. | ||
| + | |||
| + | '''(v) Re-carbonation'''<br> | ||
| + | After lime and/or soda ash treatment is applied, the treated water will generally | ||
| + | have a pH greater than 10. In addition, after softening, water becomes | ||
| + | supersaturated with calcium carbonate. If this water is allowed to enter the | ||
| + | distribution system in this state, the low pH would cause corrosion of pipes and | ||
| + | the excess calcium carbonate would precipitate out, causing scale. So, the water | ||
| + | must be re-carbonated, which is the process of stabilizing the water by lowering | ||
| + | its pH and precipitating out excess lime and calcium carbonate. | ||
| + | Therefore, the goal of re-carbonation is to produce stable water. Stable water has | ||
| + | a calcium carbonate level, which will neither tend to precipitate out of the water | ||
| + | (causing scale) nor dissolve into the water (causing corrosion). This goal is usually | ||
| + | achieved by pumping CO2 into the water. Enough CO<sub>2</sub> is added to reduce the pH | ||
| + | of the water to less than 8.7. When CO<sub>2</sub> is added, the excess lime will react with | ||
| + | CO<sub>2</sub> producing CaCO<sub>3</sub> (s). Recarbonation also lowers the water pH. | ||
| + | |||
| + | '''(b)Water Softening through Ion-Exchange'''<br> | ||
| + | The ion-exchange process is the reversible interchange of ions between a solid | ||
| + | ion exchange medium and a solution and is used extensively in industrial water/ | ||
| + | softening. The hardness producing ions preferentially replace the cations in the | ||
| + | exchangers and hence this process is also known as base or cation exchange | ||
| + | softening. | ||
| + | |||
| + | The ion exchange works on the hydrogen or sodium cycle. The hydrogen ions | ||
| + | are released into the water in the former case and the sodium ions in the latter. | ||
| + | There is only a temporary change in the structure of the exchange material. | ||
| + | The exchange material can be re-generated using acid and sodium chloride | ||
| + | respectively. | ||
| + | |||
| + | In general, ion exchange materials used in water softening, also called zeolites, | ||
| + | are hydrated silicates of sodium and aluminum. There are inorganic and organic | ||
| + | zeolites: | ||
| + | |||
| + | '''(i) Inorganic Zeolites'''<br> | ||
| + | Natural inorganic zeolite is available as ‘green sand’ while the synthetic or gel | ||
| + | type is obtained through the reaction of either sodium aluminates or aluminium | ||
| + | and is graded to suitable sizes by the reaction of either sodium aluminate or | ||
| + | aluminium sulphate with sodium silicate which, after drying, is graded to suitable | ||
| + | sizes by screening. For regeneration, 3.5 to 7 kg of salt is required for every | ||
| + | kilogram of water hardness removed. | ||
| + | |||
| + | '''(ii) Organic Zeolites'''<br> | ||
| + | These consist of carbonaceous materials and sulphonated stayrone type resins | ||
| + | which have excellent cation exchange properties, requiring for regeneration, 2-4 | ||
| + | kg salt for every kilogram of hardness removed. These are resistant to attack by | ||
| + | acid solutions and hence can be regenerated by acid. They can be used for waters | ||
| + | with a wide pH range, whilst the loss due to attrition is negligible compared to the | ||
| + | synthetic inorganic zeolites. | ||
| + | |||
| + | (iii) Raw water characteristics<br> | ||
| + | Raw water to be treated by ion exchange should be relatively free from turbidity | ||
| + | otherwise the exchange material gets a coating that affects the exchange | ||
| + | capacity of the bed. The desirability of using filters prior to zeolite beds or resorting | ||
| + | to more frequent regeneration would depend upon the level of turbidity. Metal | ||
| + | ions like iron and manganese, if present, are likely to be oxidized and can coat | ||
| + | zeolites, thus deteriorating the exchange capacity steadily since the regeneration | ||
| + | cannot remove the coats. | ||
| + | |||
| + | Oxidizing chemicals like chlorine and carbon dioxide as well as low pH in the | ||
| + | water will have a tendency to attack the exchange materials particularly the | ||
| + | inorganic type, the effect being more pronounced on the synthetic inorganic | ||
| + | zeolites. Waters low in silica inorganic zeolites, are to be avoided in boiler feed | ||
| + | water. The organic zeolites, operating on a brine regeneration cycle do not add | ||
| + | any silica to the water and consequently are ideally suited for boiler feed water. | ||
| + | |||
| + | '''Caution'''<br> | ||
| + | The ion exchange process is both costly and delicate and should not be adopted | ||
| + | without advice from a competent authority. In case the need arises for using this | ||
| + | type of process for water softening then the details of the process design should | ||
| + | be obtained from a standard textbook or plant manufacturer. | ||
| + | |||
| + | === Defluoridation of Water === | ||
| + | ==== Fluorides ==== | ||
| + | Fluoride is the ionic form of fluorine. Fluorides are organic and inorganic compounds | ||
| + | containing the element fluorine. As a halogen, fluorine forms a monovalent ion | ||
| + | (−1 charge). Fluoride forms a binary compound with another element or radical. | ||
| + | Examples of fluoride compounds include hydrofluoric acid (HF), sodium fluoride | ||
| + | (NaF) and calcium fluoride (CaF2), and uranium hexafluoride (UF6). | ||
| + | |||
| + | Fluoride compounds, usually calcium fluoride, are naturally found, usually in low | ||
| + | concentrations in water. However, water from underground sources can have | ||
| + | higher levels of fluoride to the level that it becomes a health hazard. | ||
| + | |||
| + | Excessive fluorides in drinking water may cause mottling of teeth or dental | ||
| + | fluorosis, a condition resulting in the coloration of the tooth enamel, with chipping | ||
| + | of the teeth in severe cases, particularly in children. With even higher levels of | ||
| + | fluorides, there are cases of fluorosis of the bony structure. | ||
| + | |||
| + | The chief sources of fluorides in nature are: | ||
| + | * fluorapatite (phosphate rock) | ||
| + | * fluorspar | ||
| + | * cryolite and | ||
| + | * igneous rocks containing fluorosilicates | ||
| + | |||
| + | A designer, in deciding on whether or not to include de-fluoridation in a water | ||
| + | supply scheme should, therefore, consider both the number of potential | ||
| + | consumers, alternative sources, the financial consequences both in capital and | ||
| + | running and whether or not there is a possibility to dilute the water containing | ||
| + | the fluoride as a means of reducing the concentration. Defluoridation technology | ||
| + | opted by the designer should ensure that the product (treated water) meets | ||
| + | relevant Tanzania Standards and other standards/guideline which may have | ||
| + | been adopted by the country. | ||
| + | |||
| + | ==== Defluoridation ==== | ||
| + | Defluoridation is necessary when the fluoride concentration is higher than | ||
| + | the acceptable limits. The following methods may be considered for attaining | ||
| + | defluoridised water standards. | ||
| + | * Desalination | ||
| + | * Additive methods | ||
| + | * Absorption methods | ||
| + | |||
| + | '''Desalination'''<br> | ||
| + | Desalination effectively removes all dissolved impurities from water. This can be | ||
| + | accomplished in one of several ways, by freezing, by distillation, by electrolysis or | ||
| + | by reverse osmosis. The cost of desalination is high although the costs of reverse | ||
| + | osmosis have fallen considerably in recent years. Nevertheless, this method is | ||
| + | more appropriate to deal with brackish or sea water although it should not be | ||
| + | entirely ruled out where there a few, if any, alternative sources and there is a | ||
| + | good supply of electricity. | ||
| + | |||
| + | '''Additive method'''<br> | ||
| + | In this method, one or more chemicals are added to water. The fluoride is then | ||
| + | absorbed and both the additive and the fluoride are consequently removed by | ||
| + | using conventional treatment processes such as sedimentation and filtration. A | ||
| + | wide variety of materials have been tried including lime, magnesium sulphate, | ||
| + | magnesium oxide, calcium phosphate, aluminium sulphate, various natural | ||
| + | earths, bauxite, sodium silicate and sodium aluminate. | ||
| + | |||
| + | Excessive lime treatment for water softening affects the removal of fluoride due | ||
| + | to its absorption by the magnesium hydroxide floc. However, sizeable fluoride | ||
| + | removal is possible only when magnesium is present in large quantities which | ||
| + | may not always be the case and magnesium may have to be supplemented in | ||
| + | the form of salts. | ||
| + | |||
| + | The initial cost and cost of chemicals is very high and the resultant sludge is | ||
| + | environmentally difficult to dispose of. | ||
| + | |||
| + | '''Absorption methods''' | ||
| + | Absorption methods employ a bed or filter of generally insoluble material through | ||
| + | which the water is allowed to percolate periodically. As it becomes saturated, with | ||
| + | the fluoride ‘removed, the absorptive media is either replaced or appropriately | ||
| + | regenerated. | ||
| + | |||
| + | (a) Materials used have included charred bone, activated alumina, activated | ||
| + | carbon, tricalcium phosphate, natural and synthetic ion-exchange materials | ||
| + | and aluminum sulfate. | ||
| + | (b) Studies elsewhere have revealed that activated carbon has a good capacity | ||
| + | to remove fluoride where the concentration is less than 10 mg/l and the | ||
| + | water is low in salinity. | ||
| + | (c) An activated carbon for fluoride removal has been developed in India by | ||
| + | carbonizing paddy husk or sawdust, digesting under pressure with alkali, and | ||
| + | quenching it in a 2% alum solution. The spent material can be regenerated | ||
| + | by soaking it in a 2% alum solution. | ||
| + | (d) Also a granular ion exchange material, ‘Defluoron 2’, which is sulfonated | ||
| + | coal operating on the aluminum cycle has been developed in India. | ||
| + | (e) The water treatment specialist M/S Degrémont recommended the activated | ||
| + | alumina in the case of the Arusha urban water supply project. | ||
| + | |||
| + | However, defluoridation must be regarded as a sophisticated process and | ||
| + | to determine suitability and quantity of chemical, pilot plant trials should be | ||
| + | conducted first. | ||
| + | |||
| + | === Water Conditioning === | ||
| + | Water conditioning entails ensuring that at the end of the treatment process | ||
| + | but just before the water is pumped or gravitated into the clear water reservoir, | ||
| + | storage tanks or the distribution network, the treated water should be neither | ||
| + | precipitative nor corrosive. Hence this may require a pH correction in either | ||
| + | direction depending on the outcome of the daily laboratory analysis results. | ||
| + | Moreover, if there will be a need to increase the pH, lime will have to be prepared | ||
| + | as a slurry for the sake of controlling the dosage and hence dosed at the point of | ||
| + | dosing the coagulants and other chemical additives. In case the pH of water has | ||
| + | to be increased, a dose of an acid would suffice. Care has to be taken against | ||
| + | introducing major shifts of pH due to the risk of making it limiting as for example | ||
| + | when Ferrous chemicals are used in coagulation of water. | ||
| + | |||
| + | === Management of Water Treatment Sludge === | ||
| + | The conventional water treatment plant involves the process of coagulation, | ||
| + | flocculation, sedimentation, filtration and disinfection. Large volumes of water | ||
| + | treatment sludge (WTS) or residues thereof are generated during the processing | ||
| + | of raw water to make it fit for drinking purposes. A typical water treatment | ||
| + | plant produces about 100,000 ton/year of sludge (Bourgeois et al., 2004). | ||
| + | Experiences elsewhere have shown that, due to the lack of sludge management | ||
| + | strategies, most of the WTPs discharge their filter backwash water and sludge | ||
| + | into nearby drains which ultimately meet the water source. Aluminium salts (e.g. | ||
| + | Al<sub>2</sub>(SO4)<sub>3</sub>.18H2O) or Iron salts (e.g. FeCl3.6H<sub>2</sub>O, FeCl<sub>2</sub>, FeSO4.7H2O) are commonly used as coagulants (Sales et al., 2011). These salts get hydrolysed in water to form their respective hydroxide precipitates. Colloidal and suspended impurities | ||
| + | such as sand, silt, clay, humic particles present in the crude water are removed | ||
| + | by charge neutralization, sweep floc mechanism and adsorption onto hydroxide | ||
| + | precipitates (Trinh and Kang, 2011). The hydroxide precipitate along with sand, | ||
| + | silt, clay and humic particles removed from the raw water mainly constitute the | ||
| + | solids present in the sludge. The moisture content of the wet sludge is generally | ||
| + | above 80 wt% (Tantawy et al., 2015). In general, this sludge is discharged directly | ||
| + | into nearby water bodies or dumped in the landfills after dewatering. | ||
| + | |||
| + | === Treatment of Water Treatment Sludge === | ||
| + | ==== Sludge Thickening ==== | ||
| + | Sludge thickening is defined as the removal of water from the sludge with the | ||
| + | aim of substantially reducing sludge volume. For example if sludge with 0.8% | ||
| + | dry solids (DS) can be thickened to 4% DS; a fivefold decrease in sludge volume | ||
| + | is achieved. The objective of sludge thickening is to produce a sludge that is as | ||
| + | thick as possible which can be pumped without difficulty and includes a relatively | ||
| + | solid free liquid supernatant. | ||
| + | |||
| + | '''(a) Gravity Thickening'''<br> | ||
| + | '''(i) Description of Unit'''<br> | ||
| + | Gravity sludge thickening is the method commonly adopted. The slope of the | ||
| + | bottom of the gravity thickeners should be carefully selected in order to facilitate | ||
| + | the flow of thickened sludge towards the center/collection pit. Gravity thickeners, | ||
| + | usually circular in shape and provided with pickets or rakes to improve the dewatering | ||
| + | of sludge. A dry solids content of 2-2.5% can be expected from gravity thickening. | ||
| + | The gravity thickening design is similar to a clarifier. Thickeners are usually circular shaped; | ||
| + | and the feed is carried out through a pipe to a central hood serving | ||
| + | as distribution and still area, with a height that has no effect on compaction or | ||
| + | compression bottom area. Except for small thickeners, static, and with hopper | ||
| + | floor, these units have a system of very strong bottom scrapers, which carry the | ||
| + | sludge to a central tank and on which pickets are installed. These vertical bars, that | ||
| + | move smoothly, enhance mass homogeneity and create preferential channels | ||
| + | enabling the disposal of interstitial water and occluded gases generated by | ||
| + | fermentation phenomena and facilitating the thickening. The supernatant liquid | ||
| + | is collected by a perimeter weir and sent to the plant head or primary treatment. | ||
| + | |||
| + | (ii) Design considerations and procedures | ||
| + | Hydraulic loading rate and solids load are the main design parameters. | ||
| + | The hydraulic loading rate is based on the real flow through the unit, that is, | ||
| + | which goes by the perimetral discharge weir(s) (outflow). | ||
| + | |||
| + | [[Image:Formula7.3&4.JPG|645px|link=Chapter_Seven:_Water_Treatment]] | ||
| + | |||
| + | Where,<br> | ||
| + | CS = solids load (kg SS/m<sup>2</sup>/h)<br> | ||
| + | Q = sludge flow to the thickening unit (m<sup>3</sup>/h)<br> | ||
| + | X = solids concentration (mg/L)<br> | ||
| + | A = horizontal thickener surface (m<sup>2</sup>)<br> | ||
| + | |||
| + | [[Image:Figure7.27.JPG|488px|link=Chapter_Seven:_Water_Treatment]] | ||
| + | |||
| + | '''Sludge Dewatering'''<br> | ||
| + | The alternatives for sludge dewatering systems are described below. Guidance | ||
| + | for the selection of an appropriate system is given in Table 7.5. | ||
| + | |||
| + | [[Image:Table7.5.JPG|643px|link=Chapter_Seven:_Water_Treatment]] | ||
| + | |||
| + | '''Sludge Drying Beds'''<br> | ||
| + | Sludge drying beds are most favoured when lands are available in close proximity | ||
| + | to the water treatment works. Areas where strong sunlight is available with | ||
| + | average annual rainfall lower than 2,200 mm are appropriate. The filtrate of | ||
| + | the sludge drying beds can be directed under gravity to sludge regulation tank | ||
| + | for subsequent thickening and should not be discharged to the environment. | ||
| + | However in areas having higher rainfall (average annual rainfall between 3000 to | ||
| + | 6000 mm) in order to achieve higher dry solid contents solar sludge drying beds | ||
| + | having a roof cover of UV protected polythene can be utilized. | ||
| + | |||
| + | '''Sludge Lagoons'''<br> | ||
| + | Lagoons may be the cheapest method of sludge dewatering but large land | ||
| + | area are required compared to mechanical dewatering techniques. However, | ||
| + | compared to conventional sludge drying beds, lagoons require considerably | ||
| + | lesser land. Lagoons can be lined or unlined or can be provided with under | ||
| + | drain arrangement for better dewatering requirements. Unlined earthen sludge | ||
| + | lagoons are more effective in dealing with large volumes of sludge from higher | ||
| + | capacity water treatment plants. However due consideration should be given | ||
| + | to the following during planning of the water treatment plant layout to locate | ||
| + | sludge lagoons: | ||
| + | * Fluctuation of groundwater table (seasonal high groundwater table should be- sufficiently below the bottom of the lagoon preferable below 2.5 m), | ||
| + | * Underlying soil characteristics should be investigated to see its suitability,soil- percolation rate between 25 to 150 mm/hr being preferred, | ||
| + | * Annual precipitation preferably to be below 3,000 mm, | ||
| + | * Access ramps to be provided for dumper/tractor/mini loader to collect driedsludge from the lagoon and final disposal. | ||
| + | |||
| + | ==== Mechanical Sludge Dewatering ==== | ||
| + | Important operational parameters to be considered in evaluation of these | ||
| + | systems are; | ||
| + | * energy consumption, | ||
| + | * required polymer dosage and | ||
| + | * separation efficiency | ||
| + | Further, mechanical dewatering systems such as; | ||
| + | * Belt Filter Presses, | ||
| + | * Filter Presses and | ||
| + | * Centrifuges should operate continuously as far as possible in order to reduce usage of treated water for the cleaning operation required at the end of each operation cycle and to optimize utilization of equipment. | ||
| + | These equipment require | ||
| + | * high skilled maintenance staff | ||
| + | * suitable polyelectrolyte with dosing arrangement | ||
| + | * electrical power for the operation of the equipment | ||
| + | |||
| + | Therefore, the process is usually attractive only in large sludge dewatering facilities | ||
| + | with incoming flow > 0.3 m3/s (25,920 m3/day). As such, correct assessment of | ||
| + | sludge generation and selection of the capacity of each unit is very important. | ||
| + | After mechanical dewatering, the sludge is generally directed through a conveyer | ||
| + | system into a skip or a hopper. The filtrate from mechanical dewatering facility | ||
| + | can be directed back to the sludge regulation tank. Most mechanical dewatering | ||
| + | equipment can achieve 15-20% DS content but the actual performance needs | ||
| + | to be verified from the manufacturers. Mechanical sludge dewatering is only | ||
| + | recommended for: | ||
| + | |||
| + | * major water treatment plants that generate large quantities of sludge, | ||
| + | * treatment plants that do not have adequate land or areas that experience average annual rainfall in excess of 3,000 mm. | ||
| + | |||
| + | ==== Backwash Water Recovery ==== | ||
| + | Backwash recovery aims at utilizing water resources to maximum potential, | ||
| + | to minimize energy consumption and thereby optimize production costs. The | ||
| + | backwash recovery process should not cause any adverse impacts on the treated | ||
| + | water quality. The possible health implications could be trace amounts of heavy | ||
| + | metals that may be present in raw water or in water treatment chemicals as | ||
| + | impurities, pathogenic microorganisms, algal toxins/THM which may be produced | ||
| + | during physical/chemical processes of the treatment works or present in raw water. | ||
| + | In this context, reuse of thickener supernatant is not recommended as about 95% | ||
| + | of the contaminants in raw water are removed from the sedimentation process | ||
| + | and hence may contain pathogenic organisms such as Cryptosporidium and Giardia | ||
| + | Lamblia which are resistant to chlorination. It is advantageous to reuse the backwash | ||
| + | water in order to conserve energy by minimizing the utilization of low lift pumps | ||
| + | and to recover 2-5% of water. Backwash water recovery has many advantages. | ||
| + | However, the introduction of backwash water recovery needs careful evaluation of | ||
| + | the raw water quality, the proposed treatment process and the cost-benefits. | ||
| + | |||
| + | Therefore, the recommended unit processes for backwash water recovery to be | ||
| + | incorporated in the sludge treatment stream are as follows: | ||
| + | * Backwashed water is first directed to backwash recovery tank (minimum two tanksü each having capacity at least to hold two backwashes) where it is allowed to settle for a selected time, | ||
| + | * After allowing for sedimentation, supernatant is gravity fed to backwash recirculation tank which is constructed with common wall to the backwash recovery tank to minimize construction cost, | ||
| + | * The gravity feeding system should comprise pontoon attachment at the the surface of the inlet pipe (which needs to be covered with suitable mesh to prevent escaping of floating debris) and the bottom end supported by a hinged bend in order to facilitate rotation of the pontoon attached inlet pipe as water level drops up to the sludge thickening zone of the backwash recovery tank. | ||
| + | |||
| + | ==== Waste from Slow Sand Filters ==== | ||
| + | During the scraping operation of the biological layer called “schmutzdecke” of | ||
| + | the slow sand filters, the removed sand can be washed using “hydro cyclone” | ||
| + | and reused when “re-sanding” of the slow sand filters is required. The dirty water | ||
| + | resulting from the hydro cyclone needs to be treated appropriately in line with | ||
| + | the disposal standards. A sludge thickener can be used if the number of filters | ||
| + | is higher and continuous type of treatment is needed as in larger scale water | ||
| + | treatment plants. If the treatment plant is of small scale, it may be sufficient | ||
| + | to have a roughing filter together with natural or constructed wetland to polish | ||
| + | further to meet the discharge standards as this type of system may be suitable | ||
| + | for intermittent operation as generally slow sand filters need to be cleaned once | ||
| + | in two to three months depending on the raw water quality. | ||
| + | |||
| + | ==== Disposal of Sludge ==== | ||
| + | The dried sludge resulting from different dewatering methods discussed above | ||
| + | should be disposed in compliance to environmental regulations. The options | ||
| + | available for disposal of sludge are:<br> | ||
| + | (a) Land Disposal<br> | ||
| + | (i) Forest<br> | ||
| + | (ii) Land Reclamation<br> | ||
| + | (iii) Landfill<br> | ||
| + | (b) Incineration<br> | ||
| + | (c) Melting<br> | ||
| + | (d) Brick and roof tile construction (after mixing with other constituents to | ||
| + | obtain the desired consistency)<br> | ||
| + | |||
| + | '''REFERENCES'''<br> | ||
| + | Eliakimu, N., R. Machunda and K. Njau (2018). “Water quality in earthen dams and potential health impacts: case of Nadosoito Dam, Tanzania.” Water Practice and Technology13(3): 712-723.<br> | ||
| + | Jordan K. Lanfair, Stephen T. Schroth, Archis Ambulkar (2020). Desalination. Retrieved from: https://www.britannica.com/technology/prechlorination <br> | ||
| + | ''Huisman (1974).https://sswm.info/sswm-university-course/module-6-disaster-situationsplanning-and-preparedness/further-resources-0/slow-sand-filtration)''<br> | ||
| + | Mbwette, T.S.A and Wegelin 1984. Field Experience with HRF-SSF Systems in Treatment of Turbid Surface Waters in Tanzania. Proc. IWSA Congress, Monathis, Tunisia pp 10-12 (SS6)<br> | ||
| + | https://www.slideshare.net/gowrivprabhu/water-treatmentwater-treatment | ||
| + | |||
| − | + | Previous Page: [[Chapter_Six:_Pumping_Systems|Chapter_Six:_Pumping_Systems]] << >> Next Page: [[Chapter_Eight:_Treatment_of_Waters_With_Special_Contaminants|Chapter_Eight:_Treatment_of_Waters_With_Special_Contaminants]] | |
| − | + | </div> | |
Latest revision as of 11:03, 17 July 2022
Contents
- 1 Chapter 7: WATER TREATMENT
- 1.1 INTRODUCTION
- 1.2 RECOMMENDED OVERALL DESIGN APPROACH FOR COMPONENTS OF WATER TREATMENT PLANTS
- 1.3 DOCUMENTS AND WEBSITES CONSULTED AND WHICH ARE HYPER-LINKED TO THE DCOM MANUAL
- 1.4 WATER TREATMENT DESIGN CONSIDERATIONS
- 1.5 WATER TREATMENT LEVELS AND UNITS
- 1.5.1 Pre-treatment
- 1.5.1.1 Scum and Floating Materials Skimmer
- 1.5.1.2 Screening or Straining
- 1.5.1.3 Grit Removal
- 1.5.1.4 Design approach
- 1.5.1.5 7.5.1.3.1 Design criteria
- 1.5.1.6 Sand Traps
- 1.5.1.7 Pre-chlorination
- 1.5.1.8 Water pre-conditioning (pH adjustment)
- 1.5.1.9 Primary Treatment
- 1.5.1.10 Sedimentation
- 1.5.1.11 Lamella Plate Settlers (Inclined plate settlers)
- 1.5.1.12 Primary Filtration
- 1.5.1.13 Slow Sand Filtration
- 1.5.1.14 Floatation
- 1.5.1.15 Dissolved-Air Floatation
- 1.5.1.16 Electrolytic Floatation
- 1.5.1.17 Dispersed-Air Floatation
- 1.5.1.18 Aeration
- 1.5.1.19 Falling Water Aerators
- 1.5.1.20 Spray Aerators
- 1.5.2 Secondary Treatment
- 1.5.3 Tertiary Treatment
- 1.5.4 Water softening
- 1.5.5 Defluoridation of Water
- 1.5.6 Water Conditioning
- 1.5.7 Management of Water Treatment Sludge
- 1.5.8 Treatment of Water Treatment Sludge
- 1.5.1 Pre-treatment
1 Chapter 7: WATER TREATMENT
1.1 INTRODUCTION
In this chapter, different categories of the unit operations that are utilized to achieve different water treatment levels are described. It is followed by description of the recommended approach of design of treatment plant components. Emphasis should be given to potential water sources that have undergone investigations on the variability of both the quality and quantity for at least two years. The data gathered should be used for selection of appropriate treatment flow sheets and designing it.
1.1.1 Classification of the qualities of water sources found in Tanzania according to the complexity of its treatment
Water treatment refers to any process that improves the quality of water to make it more acceptable for human consumption. The production of drinking water involves the removal of contaminants from raw water to produce water that is pure enough for human consumption without any short-term or long-term risk of any adverse health effects.
The processes involved in removing contaminants from water includes physical processes such as settling and filtration, chemical processes such as disinfection and coagulation and biological processes such as slow sand filtration. Table7.1 present the recommended treatment process flow for the most common water sources in Tanzania. The above water contaminants removal processes are best presented in water treatment flow sheets (Figures 7.1–7.8).
1.1.2 Classification of Unit Operations to Achieve Water Treatment Levels
Categories for water treatment levels are; pre-treatment, primary treatment, secondary treatment and tertiary treatment.
- Pre-treatment includes units like Scum and floating matters removal, Screening (fine and coarse), Sand trap, Grit removal, Pre-chlorination, Water conditioning (pH correction).
- Primary treatment comprises of Sedimentation, Primary filtration, Floatation, Aeration.
- Secondary treatment includes Coagulation, Flocculation, Clarification, Filtration, Softening, Reverse Osmosis, Capacitive De-Ionisation (CDI), Ion Exchanger, Adsorption, Constructed wetlands.
- Tertiary treatment includes Disinfection, Softening, Water conditioning, Water polishing.
1.2 RECOMMENDED OVERALL DESIGN APPROACH FOR COMPONENTS OF WATER TREATMENT PLANTS
The design of the main conveyance units for the treatment plants including pipes and channels should be designed for a period of 20 years (design life). On other hand, the individual unit operations should be designed for a design period of 10 years in an approach that allows for phased implementation. Intentionally, to allow for adoption of the latest technologies and to avoid tying substantial capital in the treatment plants. It is recommended that a potential water source should be closely and intensely monitored for a period ranging from 2 to 3 years. This period can include the time when the feasibility study for the water supply project is being undertaken including the environmental impact assessments. Water source quality and quantity data should be used for determination of the most suitable treatment flow sheet.
1.3 DOCUMENTS AND WEBSITES CONSULTED AND WHICH ARE HYPER-LINKED TO THE DCOM MANUAL
The following documents were consulted for purposes of making reference for the design of the treatment plants in Tanzania:
- URT, 2009. Design Manual for Water Supply and Wastewater Disposal.
- Ministry of Drinking Water and Sanitation, May 2013. Operation and maintenance manual for rural water supplies. India.
- The Republic of Uganda, Ministry of Water and Environment, 2013. Water Supply Design Manual, 2nd edition.
- World Bank Philippines, February 2012. Water Partnership Program. Rural Water Supply Vol.I Design Manual.
- Washington State Dept. of Health USA, October 2019. Water System Design Manual.
- URT, July 1997. Design Manual for Water Supply and Wastewater Disposal.
Throughout this manual, on a number of occasions the designers are referred to the websites of the Ministry of Water (https://www.maji.go.tz) or RUWASA (https://www.ruwasa.go.tz) vide the various hyperlinks inserted in order to access the standard drawings for various appurtenances.
1.4 WATER TREATMENT DESIGN CONSIDERATIONS
The manual has made reference to a number of design guidelines that are relevant for the design of various unit operations and wherever necessary the full guidelines have been hyper-linked to the manual. For each unit operation, a few critical design criteria have been provided.
Before proposing or designing any treatment plant for any planned water supply project, water quality of the anticipated water sources to be treated has to be known to the designer. Knowing the historical and current water quality trends of the sources will help in designing a treatment plant that can address the localized water quality challenges of concern in the given area apart from the general water quality parameters.
The sizing and selection of the treatment technology and different units to be installed should always aim at meeting the established national and international water quality standards and associated health criteria which are often updated from time to time. There are different criteria and standards across the world, however, in Tanzania, the most recent standards of Tanzania Bureau of Standards and the World Health Organization (WHO) guidelines are the guiding documents recommended to be referred when designing a water supply project.
1.5 WATER TREATMENT LEVELS AND UNITS
1.5.1 Pre-treatment
1.5.1.1 Scum and Floating Materials Skimmer
This is the unit operation that enables the manual or automated removal of scum and floating matter ahead of the screening units. These are designed to skim the entire width of the approach area ahead of the screens. In view of the variability of flow of water from the sources, skimmers ought to be designed such that they can be adjusted up or down depending on the quantity variation that is established during the feasibility study. The width of the channel or any open conduit delivering the raw water will determine its design. Figure 7.9 shows the design of such a skimmer.
1.5.1.2 Screening or Straining
This unit operation consists of fine screens and coarse screens which perform the task of removing all fine and coarse matters that may block the screen or damage downstream appurtenances or machines. This is a physical, pretreatment process used to remove weeds, grass, twigs, bilharzial snails and other freshwater crustaceans as well as coarser particles including plastics, tins and other hard matter so that they do not enter the pumping, treatment, or supply system. Screens are placed at the entrance to the intake of a water supply project.
The design considerations for surface water screens are;
- They should be easily accessible, at least during medium and low flows and inclined downstream of the river or stream as well as during cleaning (if manually done) as indicated in Fig.7.10.
- Distance between bars should be between 10 and 30 cm. for coarse screens and between 0.5 and 5 cm. for fine screens. The shape of the screen bars is either round or rectangular.
- Approach velocity entering the screen (Va) from upstream should not exceed 0.3 to 0.5 m/sec. to limit sedimentation.
- Velocity through the screens (Vs) should not exceed 0.7 to 1.0 m/sec. to prevent soft deformable materials from being forced through the screens.
- The ratio of the width of the screens (Ø) and the space between the bars (b) determines the ratio between the two velocities (Va) and (Vs).
- Small screens are made removable for cleaning, medium-sized can be hand raked in-situ whilst large screens will need in-situ mechanical or electrical operated rakes.
Figure 7.10 presents the formula for the calculation of the headloss through the screens as well as the screen bars coefficient (ƥ).
1.5.1.3 Grit Removal
Grit consists of the heavy inorganic fraction of sewage solids that includes road grit, sand, egg shells, broken glass, coconut shells and metal pieces. The purposes of including grit channels in the design are as follows:
- To protect pumps and other mechanical parts from excessive wear and tear,
- To avoid undue clogging/filling up of subsequent unit operations,
- To differentially remove grit but not the organic particulates in water.
The average specific gravity of grit is 2.5 with an average settling velocity S = 30 mm/sec. In comparison, while sand grit has an average solids density ƥs = 2650 kg/m3organics have a density ƥo ranging from 1020 to 1200 kg/m3.
1.5.1.4 Design approach
To exploit the differential sedimentation rates of the particles by providing channels that ensure removal of grit rather than any other lighter particles and to maintain the horizontal flow velocity Vh has to be maintained at about 0.3 m/sec. Provision of a parabolic or near parabolic cross section of the channel guarantees that the constant velocity is maintained at all flows. In practise, due to the difficulty of construction of parabolic sections, trapezoidal sections are used.
1.5.1.5 7.5.1.3.1 Design criteria
Length of the channel L = 20 (maximum depth of flow)
L/d = Vh/Vs Where, Vh = Horizontal flow velocity Vs = Vertical settling velocity L = Length of the grit channel D = Depth of flow in the channel
There are three types of grit channels that can be designed, these include horizontal flow, Rotational flow, and Vertical flow
1.5.1.6 Sand Traps
This pre-treatment unit is designed to trap sand after water has been guided into the intake chamber in order to reduce wear and tear as well as silting up the unit operations that are located downstream of the intake structure. The minimum diameter of washout pipes of such sand traps is 75 mm and the bigger the main intake pipe, the bigger is the flushing pipe for sand. Figures 7.15, 7.16 and 7.17 show the layout plan and two sections of such a sand trap for small streams intake.
1.5.1.7 Pre-chlorination
This is a unit operation that is used for purposes of controlling algae growth in raw water and the process of preparation of the chemical to facilitate dosing will include the standard preparation of the aqueous solution as done during disinfection. This is often added upon establishment of occurrence of algal blooms during certain periods of the year as confirmed by laboratory tests undertaken daily. The amount of the dose will be established daily in order to pre-determine the pre-chlorination dose that has to ensure no interference with downstream unit operations in case the flow sheet involves biological treatment processes like slow sand filter or others. The location of dosing of the chemical has to be secured against direct sunlight and has to ensure intense mixing. Where chlorine gas is used for pre-chlorination, the usual measures that are taken when dosing chlorine in gaseous form have to be taken including leakage detection. To minimise chlorine consumption, pre-chlorination has to be done downstream of the fine screens.
Other design features can be seen in the Appendix H. of this DCOM Manual. It should be noted that pre-chlorination also controls growth of bacteria in pipes and tanks (BRITANICA, 2020). Lanfair et al (2020) cautions that if pre-chlorination is applied to water with a high concentration of natural organic matter (nom), the latter is suspected to react with chorine to form Disinfection by-products (DBPs) that include trihalomethanes or haloacetic acids that are suspected to contribute towards stomach and bladder cancer.
1.5.1.8 Water pre-conditioning (pH adjustment)
Water pre-conditioning can entail a number of pre-treatments undertaken prior to pre- chlorination which is often the final step in pre-treatment. This unit operation involves adjustment of the pH upstream in order to ensure the chemicals used during further treatment processes are dosed to water that has the correct pH range for maximum efficiency. A good example is when the treatment flow sheet includes coagulation with Ferrous Sulphate that requires an optimum pH range of 7 to 8.5. As a matter of fact,the application of this unit operation guarantees that the daily fluctuations in quality are also reflected in the chemical that is used for pH correction whether an acid or an alkali is used. It is usual to apply lime for increasing pH and use of acids like dilute sulphuric acid or hydrochloric acid. Other pre-conditioning measures include use of sodium carbonate (soda ash) to remove hardness (i.e. calcium carbonate).
1.5.1.9 Primary Treatment
Primary treatment of water removes material that will either float or readily settle out by gravity. This water treatment level includes the physical processes of screening, grit removal and sedimentation.
1.5.1.10 Sedimentation
In designing sedimentation tanks, the required detention time determines the dimensions of the tank. A rectangular tank is the simplest design to use. Detention time is calculated as Volume/Flow rate (Q). The detention time based on the average daily flows usually ranges from about 45 minutes to 3 hours depending on water turbidity. The ideal inlet reduces the entry velocity and distributes the water as uniformly as possible across the depth and width of the tank. Outlets are usually weirs that are sufficiently long to reduce the flow velocity, and so avoid the re-suspension of the solids in the water. Plain sedimentation tanks should be designed for a surface loading in the range of 0.1 - 0.5 m3/m2/h. The exact surface loading to be adopted should be determined after carrying out settlement tests on samples of raw water, typical of all regimes of the water source. The settling properties of water will depend on the soil and vegetation conditions in the catchment area, and they will vary considerably between different locations and regimes of the water source. Figures 7.18 (a and b) show a cross-section through a circular sedimentation tank while Figures 7.19 (a) and (b) show a cross-section through a rectangular sedimentation tank.
1.5.1.11 Lamella Plate Settlers (Inclined plate settlers)
Settling efficiency of a basin depends upon the design surface loading. Lamella plates and small diameter tubes having a large wetted perimeter, relative to wetted area providing laminar flow conditions and low surface loading rates have shown good results in terms of settling efficiency and economy in space as well as cost. Plate or tube configuration can be horizontal or steeply inclined. In inclined plates or tubes (55o – 60o) continuous gravity drainage of the settleable material onto the floor below can be achieved, without impairment of effluent quality. However, to work effectively an efficient flocculation stage is critical.
In purpose-built lamella plate settlers, the water enters at the base of the lamella plates and travels upwards between the lamellas. Each space between the lamella plates tends to act as a semi-independent settling module with the lamella plates extending from near the base of the tank to about 125 mm above the top water level. The clarified water is collected by saw-toothed notched launders running along each side of the plate. Unless sludge is removed mechanically by a scraper, sufficient depth beneath the plates is required for access during cleaning, although this can be aided by pressurized water. A typical arrangement is illustrated in Figure 7.20.
top water level. The clarified water is collected by saw-toothed notched launders running along each side of the plate. Unless the sludge is removed mechanically by a scraper, sufficient depth beneath the plates is required for access during cleaning, although this can be aided by pressurized water. A typical arrangement is illustrated in Figure 7.20.
Plates are made of stainless steel or plastics with a width of 1.25 to 1.5 m and a length of 2.5 to 3.25 m including the length above water. It is advisable to use standard lengths of metal sheets and plastic sheets available in the market. In Tanzania sheets are in dimensions of 120 cm x 240 cm. Plate thickness is usually about 0.7 mm for stainless steel whilst the horizontal spacing between plates is varied according to the nature of the raw water but within the range 50 – 80 mm.
Total settled area is then:
A = (n-1) × L × W × cos θ (7.2)
Where; n = number of plates L = plate length in water (m), less the transition length W = the plate width (mm), and θ = the angle of inclination of the plates to the horizontal (55o – 60o) A = Settled area (m2)
1.5.1.12 Primary Filtration
Primary filtration of water removes material whose particle size is greater than the opening size. The target particles are those which are not removed by sedimentation tanks.
1.5.1.13 Slow Sand Filtration
A Slow Sand Filter (SSF) is basically a large tank containing the sand bed. A distinguishing feature of a slow sand filter is the presence of a thin layer, called the schmutzdecke, which forms on the surface of the sand bed and includes a a large variety of biologically active micro-organisms.
Water is introduced at the top and trickles down through the sand bed to the under-drains and goes to the storage tank. The impurities in the water are retained at the upper layers of the sand bed. In the process, the schmutzdecke consisting of bacteria and microscopic plants which grow. The schmutzdecke removes the organic matter and most of the pathogenic micro-organisms in water which might be smaller than the pores of the sand.
(a)Elements of a Slow Sand filter
Figure 3.43(a) presents, in diagrammatic form the various elements that go to make up a Slow Sand Filter. Essentially the SSF elements consist of:
(i)a supernatant (raw) water reservoir, the principal function of which is to maintain a constant head of water above the filter medium, this head providing the pressure that carries the water through the filter;
(ii)a bed of filter medium (nearly always sand), with in and upon which the various purification processes take place
(iii)an under-drainage system, which fills the dual purpose of supporting the filter medium while presenting the minimum possible obstruction to the treated water as if emerges from the underside of the filter-bed; and
(iv)a system of control valves to regulate the velocity of flow through the bed, to prevent the level in the raw water reservoir from dropping below a predetermined minimum during operation, and to permit water levels to be adjusted and backfilling to take place when the filter is put back into operation after cleaning
(v)It comprises approximately 1.2 m depth of fine sand supported on two or three gravel layers. The effective size of the sand used in slow sand filters is about 0.2 mm, but may range between 0.15 mm and 0.35 mm, and with a coefficient of uniformity of between 1.5 and 3.0.
(vi)It is a very simple and effective technique for purifying surface water. It will remove practically all of the turbidity from the water as well as most of the pathogens without the addition of chemicals. Slow sand filters can frequently be constructed largely from locally-available materials.
In an SSF the water is purified by slow percolation through a bed of fine sand. Pre-treatment is necessary with raw waters having average turbidity of 25 NTU or more, but should be considered also for less turbid raw waters 5 to 25 NTU) to improve effluent quality and reduce the frequency of cleaning. The SSF is also useful for treating groundwater containing solids in suspension, e.g. ferric and manganese compounds converted by aeration from the soluble state of the salts.
(b)Design Considerations of Slow Sand Filter (SSF)
- Raw water quality and necessity for pre—treatment and/or aeration;
- Necessity for chlorination room and clear water storage, pumping and distribution;
- Site location, foundation conditions, space for expansion and pre-treatment;
- Availability of source of filter media, and construction materials;
- Fencing and security
- The filter sand must be free from any clay or silt content and preferably of a well- rounded quartz material. Organic matter should be avoided.
(c)Design Criteria;
- When choosing the filter sand, the grain size distribution should meet the effective size 0.15 to 0.35 mm and the coefficient of uniformity should be less than 3 ,
- In order to calculate for the total area of filter beds, a working rate of 0.1 - 0.2, m3/m2/hr is recommended. When one filter is not operational, the working rate of the remaining filter should not exceed 0.2 m3/m2/h.
- The turbidity in the incoming water should not exceed an average of 5 - 10 NTU. In cases of higher turbidity, preliminary treatment such as roughing filters is necessary
- The inlet structure should be designed in such a way that the raw water is equally distributed over the filter bed area.
- To achieve this, the inlet velocity should be around 0.1 m/s and the width of the inlet structure should be at least (0.05 x Q) meters, where Q is the design flow in m3/h.
- The minimum size of a filter unit should be 15 to 20 m2;
- The height of the supernatant water should be 1 to 1.5 m,
- The oxygen content of the water after filtration should not be less than 3 mg/l.
- Calculate filter surface area = flow capacity (m3) / rate of filtration
(d)Main Water Under-drain
- Calculate Diameter of the under drain = (22 × d2) / (28 × r)
- Area of holes or slots to be 1.5% of area of the filter
- As a check, the oxygen content of the water after filtration should not be less than 3 mg/l.
- Freeboard above water level = 0.2 - 0.3 m
- Height of walls above ground surface= > 0.8 m
- Gravel filter support = 0.3 - 0.5 m
- Depth of under—drainage system = 0.3 - 0.5m
- Area (A) per filter bed = 10 - 100 m2
Rapid Gravity Sand Filtration
This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media that actually removes the particles from the water. The filter media is routinely cleaned by means of a backwashing process.
Rapid Sand Filtration (RSF) is a technique commonly used for treating large quantities of drinking water. It is a relatively sophisticated process usually requiring power- operated pumps for backwashing or cleaning the filter bed, and some designs require flow control of the filter outlet. A continuously operating filter will usually require backwashing about every two days or so when raw water is of relatively low turbidity and at least daily during periods of high turbidity. Because of the higher filtration rates, the area required for a rapid gravity filtration plant is about 20% of that required for slow sand filters.
Surface loading should be between 4 and 7 m3/h.m2, and the filter structure should be designed with a minimum height between the top of the filter media and the bottom of the wash water channel of at least 30% of the height of the filter media as this expands during backwashing. It may be necessary to include for air-scour as well as backwashing, or for the two combined in a single operation.
Normally a sufficient distribution of the wash water will be achieved if:
- Ratio of area of orifice to area of bed served (1.5 to 5) × (103) : 1
- Ratio of area of main to laterals served (1.5 to 3) : 1
- Diameter of orifices 5 - 20 mm
- Spacing of orifices 100 - 300 mm centre to centre
- Spacing of laterals approximating to the spacing of orifices.
The filter bed should be approximately 1.0 m thick and preferably consist of well- rounded quartz sand with an effect size of 0.7 - 1.0 mm and uniformity coefficient in the range of 1.3 - 1. 5. The available hydraulic head above the top of the filter bed should be 1.3 - 1.5 m. The following stratification of the sub structure should be used to support a filter of an effective grain size as suggested earlier (finest strata at the top).
- 0.15 m of grain size 2 - 2.8 mm
- 0.10 m of grain size 5.6 - 8 mm
- 0.10 m of grain size 10 - 20 mm
- 0.10 m of grain size 20 - 40 mm
- 0.10 m of grain size 40 - 60 mm
The washing velocity should be in the range of 35 – 55 m3/m2/hour, however care must be taken to ensure that sand carry over into the wash-water channel does not occur and the actual wash-water rate adjusted accordingly.
In order to achieve proper washing of the filter a storage volume sufficient for continuous washing for an 8 to 10 minute period should be available.
(b)Design Steps
The following design steps should be followed;
(i)Filter Units
- Rate of filtration should be 4 - 6 m3/m2/hr
- Determine flow capacity in the filter (m3/day. demand)
- Calculate filter surface area = flow capacity (m3) / rate of filtration
(ii)Main Water Under-drain
- Select flow capacity and flow velocity
- Calculate area required = (low capacity)/ (low velocity) (m2)
- Calculate diameter of the underdrain, d= Square Root of (4A/Π)
- Area of holes = 0.2 - 0.4 % of the area of filter
- Use five layers of sand particles in the filter as indicated above:
- Water depth 1 – 1.2 m
- Sand depth 1 – 1.2 m
(c)Filter Backwashing
A major cause of poor performance by rapid gravity filters is a result of either inadequate or excessive backwashing rates. Backwashing is sometimes carried out by water alone but more often by air and water usually applied one after the other by reverse flow to the filter bed. The first operation however is to allow the filter to drain down until the water lies a few centimetres above the top of the bed. Air is then introduced through the collector system at a rate of about 6.5 to 7.5 mm/s.
Where air and water is applied separately, air scour normally lasts about 3 – 4 minutes and the water wash about 4 – 6 minutes. Where applied concurrently, air is first introduced and after about 1.5 – 2 minutes when it is fully established water is introduced and the combined backwash last for about 6 – 8 minutes. Air is stopped first and the water run for several more minutes to rinse the bed. Generally, total water consumption per wash amounts to about 2.5 bed volumes, but should normally not exceed 2% of the treated water output in well run plants.
Comparison between Slow Sand Filters and Rapid Sand Filters
a)Base material: In SSF it varies from 3 to 65 mm in size and 30 to 75 cm in depth while in RSF it varies from 3 to 40 mm in size and its depth is slightly more, i.e. about 60 to 90 cm.
b)Filter sand: In SSF the effective size ranges between 0.2 to 0.4 mm and uniformity coefficient between 1.8 to 2.5 or 3.0. In RSF the effective size ranges between 0.35 to 0.55 and uniformity coefficient between 1.2 to 1.8.
c)Rate of filtration: In SSF it is small, such as 100 to 200 L/h/sq.m. of filter area while in RSF it is large, such as 3000 to 6000 L/h/sq.m. of filter area.
d)Flexibility: SSF are not flexible for meeting variation in demand whereas RSF are quite flexible for meeting reasonable variations in demand.
e) Post-treatment required: Almost pure water is obtained from SSF. However, water may be disinfected slightly to make it completely safe. Disinfection is a must after RSF.
f)Method of cleaning: Scrapping and removing of the top 1.5 to 3 cm thick layer is done to clean SSF. To clean RSF, sand is agitated and backwashed with or without compressed air.
g)Loss of head: In case of SSF approx. 10 cm is the initial loss, and 0.8 to 1.2m is the final limit when cleaning is required. For RSF 0.3m is the initial loss, and 2.5 to 3.5m is the final limit when cleaning is required.
Other Types of Filters
(a)Pressure Filters
Pressure filters are circular pressurized vessels containing the filter media and usually designed for vertical flow. They work on the same principle as rapid gravity filters differing in that the filter medium is enclosed in a steel vessel and the water is forced through it under pressure.
(b)Upward Flow Filters
Upward flow filters are theoretically more efficient than gravity filters where the water to be filtered flows upwards through the naturally desegregated, progressively finer and finer media so that coarser particles are trapped first in the coarser bottom layers. This tends to extend the period between backwashing. Several sand grades, getting progressively finer upwards have also been used in which case some restraining means such as a grid is required often located about 0.1 m below the surface where upward backwashing is used.
The major reason why RSF is a preferred option of water filtration to SSF is the rate of filtration where RSF is higher per filter area flexibility, where SSF is not flexible for meeting variation in demand, method of cleaning wherein the case of RSF, no sand is lost. Also, RSF requires minimal land requirements (Source: Ugandan Water Supply Design Manual (2013).
(c)Roughing Filters
Roughing filters have their place as a form of primary treatment, especially for turbid or highly changeable river water. This technique of primary treatment has been greatly under-utilised in Tanzania in the past. Although much research has been undertaken since late 1970s (Mbwette & Wegelin, 1984). It is used primarily to remove solids from high turbidity source waters prior to treatment with such unit operations as slow sand filters.
In a typical roughing filter, there are series of tanks which are filled with progressively smaller diameter media in the direction of the flow which can either be horizontal or vertical. With respect to the vertical flow direction, up-flow or down-flow roughing filters can be designed. The media in the tank which may include gravel, rice husks or any other suitable local material, plays an important role in reducing the vertical settling distance of the particles to a distance of a few millimeters
(i)Advantages of Roughing filters
- Can considerably reduce the number of pathogens in the water, as well as the amount of iron and manganese.
- Can be considered a major pre-treatment process for turbid surface water since they efficiently separate fine solid particles over prolonged periods.
- Long filters (10 m) at low filtration rates (0.5 m/h) are capable of reducing high suspended solids concentrations (1000 mg/l TSS down to less than 3 mg/l).
- Capable of reducing peak turbidities by 80 to 90 percent and fecal coliforms by 77 to 89 percent.
- They are placed at the treatment plant site and operated in combination with other pre-treatment units such as dynamic filters or sedimentation tanks.
NB: For detailed information, designers are recommended to download the comprehensive SANDTEC design report entitled ‘Surface Water Treatment by Roughing Filters, A Design, Construction and Operation Manual’ available at http// sandec.ch/WaterTreatment/Documents/Surface%20Water%20Treatment.pdf.
Features of Roughing Filters (RF).
The main part of the filter is the section containing the filter material. However, a Roughing filter comprises the following six elements:-
- Inlet Flow Control
- Raw Water Distribution
- Actual Filter
- Treated Water Collection
- Outlet Flow Control
- Drainage System
(ii)Parameters for Design of Roughing Filter
- Filter media size
- Filtration Rates
- Filter Length
- Filter media materials
(iii)Filter Media Size
- The size of filter media decreases successively in the direction of water flow and ideally the uniformity of filter media fractions is maximized to increase filter pore space (storage capacity) and aid in filter cleaning (Boller, 1993).
- The use of multiple grades of filter media in a roughing filter promotes the penetration of particles throughout the filter bed and takes advantage of the large storage capacities offered by larger media and high removal efficiencies offered by small media
- The effect of surface porosity and roughness of filter media on particle removal efficiency in roughing filtration is insignificant compared to the size and shape of macro-pores in the filter (Wegelin, 1986)
- Media types commonly used in roughing filtration are quartz, sand and gravels but can be replaced by any clean, insoluble and mechanically resistant material (Graham, 1988)
- Common grades of media used in roughing filters are provided in the Table 7.4
(iv)Filtration Rates
- Filtration rate has a significant influence on the treatment removal.
- Good removal in roughing filters are best achieved with low filtration rate (Boller, 1993), because low filtration rates are critical to retain particles that are gravitationally deposited to the surface of the media.
- While used as pretreatments for iron and manganese removal, it can operate at filtration rates of 1.5 - 3 m/h (Hatva, 1988). It is reported that horizontal flow roughing filter is capable of removing metals like iron, manganese, turbidity, and color at a filtration rate of 1.8 m/h (Dastanaie, 2007)
- At increased filtration rates (2 m/h), coarse particles penetrate deeper into the bed and they cause a decrease in filter efficiency (Wegelin et al. (1986). Whereas at 1 m/h there is good distribution of solids loading throughout the bed. Hendricks (1991) also suggested that normal filtration rate of horizontal roughing filters is between 0.3 and 1.5 m/h.
(v)Filter Media Length
- Improved cumulative removal efficiencies are typically correlated to longer filter lengths (Collins, 1994; Wegelin, 1986).
- Incremental removal efficiencies decrease with increasing filter length due to the preferential removal of larger particles early in the filter (Wegelin, 1996).
- The rate of decline is dependent on filter design variables and the size and nature of particles in suspension. The use of different media sizes may allow for treatment targets to be met by a shorter filter with multiple media sizes compared to with long filter packed with one media size.
(vi)Filter Media Materials
The following material could therefore be used as filter media:
a)Gravel from a river bed or from the ground.
b)Broken stones or rocks from a quarry.
c)Broken burnt clay bricks.
d)Plastic material either as chips or modules (e.g. used for trickling filters) may be used if the material is locally available.
e)Burnt charcoal, although there is a risk of disintegration when cleaning the filter material, should only be considered in special cases (e.g. for removal of dissolved organic matter).
f)Coconut fiber, however, due to the risk of flavoring the water during long filter operation, should be used with care.
g)Broken burnt bricks and improved agricultural waste (e.g. charcoal, maize cobs), can also be effectively used as pretreatment media (Ochieng (2006) and therefore could serve as alternatives where natural gravel is not readily available.
(vii)Types of Roughing Filters
There are many types of roughing filters with different flow directions and with different types of filter medium (e.g. sand, gravel, coconut husk fiber). However, the common types are:
i.Horizontal Flow Filters (HRF)
ii.Vertical (VRF)
iii.Dynamic (DRF)
(d) Bank Filtration (BF) Bank filtration (BF) is the infiltration of surface water, mostly from a river system into a groundwater system induced by water abstraction close to the surface water (e.g. river bank). This water abstraction is commonly done by operating wells.
As the water flows through the soil, it is filtered and hence its quality is improved. In the context of developing or newly industrialized countries, bank filtration may contribute to a more sustainable water cycle by recharging stressed groundwater bodies with filtered surface water. (Sharma & Amy 2009; Huelshoff et al.)
Bank filtration is a water treatment technology that consists of extracting water from rivers by pumping wells located in the adjacent alluvial aquifer. During the underground passage, a series of physical, chemical, and biological processes take place, improving the quality of the surface water, substituting or reducing conventional drinking water treatment.
Bank filtration works by pumping pressure in the alluvial aquifer adjacent to the river that forces the water to percolate from the river into the aquifer. In this path, a series of physical and biogeochemical processes take place, including physical filtration, adsorption, absorption, biodegradation, and dilution. Thus, riverbank-filtrate often shows better quality than river water, making its treatment for human consumption easier and less expensive.
The removal of sediment, organic and inorganic compounds, and pathogens takes place during the first metres from the river in what is known as the hyporheic zone, which usually presents reducing conditions due to the high microbial activity which consumes the oxygen in the water. Within this zone there are important biochemical processes and Redox reactions that affect groundwater quality.
The efficiency of BF depends on the local conditions including the hydrology and hydrogeology of the site, the geochemistry of water (from both the river and the aquifer), the geochemistry of microbial populations, and associated metabolic activity. This is the reason as to why it is difficult to define general procedures for identifying appropriate sites to implement the BF technique, as well as the expected efficiency of the process.
One limitation on the efficiency of BF is the clogging of the bed and the banks of the river, which decreases the hydraulic conductivity in the hyporheic zone. This clogging can be caused by infiltration of the fine sediments, gas entrapment, bio-film formation related to microbiological activity, or the precipitation and co-precipitation of inorganic compounds, being the first of these the most influential factor in clogging formation.
Siting and Design Parameters
- The most important parameters for success during BF are the flow path length, the thickness of the aquifer, and the infiltration area in the river (Grischek et al. (2002).
- The siting and design of a BF system depend on hydrogeological, technical, economical, regulatory, and land-use factors.
- Riverbank filtration wells can be designed either vertically (as the most common practice especially for the extraction of low water quantities) or horizontally (for higher extraction rates).
- Local factors such as river hydrology, hydrogeological site conditions (i.e., aquifer thickness and hydraulic conductivity), and the aims of water withdrawal are used to determine the capacity of the wells, the travel time of the bank filtrate, distance between the river and the well.
- Horizontal wells (sometimes with a radial pattern), also known as collector wells, are directed toward the river and extract water from beneath the riverbed, whereas vertical wells extract water along the riverbed.
- The BF wells can also be distributed parallel to the riverbank in galleries or groups
1.5.1.14 Floatation
Floatation may be defined as the transfer of a suspended phase from the bulk of a dispersion medium to the atmosphere/liquid interface by means of bubble attachment. There are three basic processes involved which are: bubble generation, bubble attachment and solids separation.
Floatation is described as a gravity separation process, in which gas bubbles attach to solid particles to cause the apparent density of the bubble-solid agglomerates to be less than that of the water thereby allowing the agglomerates to float to the surface. The different methods of producing the gas bubbles give rise to different types of Floatation processes which are Dissolved-air Floatation, Electrolytic Floatation and Dispersed-air Floatation.
1.5.1.15 Dissolved-Air Floatation
This is a relatively new solution for the clarification of surface and ground waters. The process as shown in Fig 7.24 is relatively simple and can be very effective. After flocculation, the produced floc attaches to micro-bubbles and rises to the water's surface. The floated solids are periodically evacuated either hydraulically or mechanically, depending on the sludge concentration required.
The dissolved air flotation process can be a good solution for the treatment of water with a high concentration of algae or other low density particles. The system is said to have a number of advantages including:
a.Reliable removal of algae, cryptosporidium and giardia cysts
b.Removal of colour and taste compounds
c.Removal of low-density solids
d.No polymer required
e.Concentrated sludge
f.Rapid start-up after shutdown
g.Few mechanical components
h.Low operating costs
The removal of suspended matter solids is achieved by dissolving air in the water under pressure and then releasing the air at atmospheric pressure in a floatation tank basin. The released air forms tiny bubbles which adhere to the suspended matter causing the suspended matter to float to the surface of the water where it may then be removed by a skimming device. Drinking water supplies that are particularly vulnerable to unicellular algal blooms, and supplies with low turbidity and high colour often employ DAF.
1.5.1.16 Electrolytic Floatation
The basis of electrolytic or electro-Floatation is the generation of bubbles of hydrogen and oxygen in a dilute aqueous solution by passing a direct current between two electrodes. Electrical power is supplied to the electrodes at a low voltage potential of 5 to 10 VDC by means of a transformer rectifier. The energy required for electro- Floatation depends largely on the conductivity of the liquid and the distance between the electrodes.
1.5.1.17 Dispersed-Air Floatation
Both foam and froth dispersed air Floatation are unsuitable for water treatment applications because the bubble size tends to be large (>1 mm. compared to 20-111 for dissolved-air Floatation and electro-Floatation) and either high turbulence (froth Floatation) which would break up the fragile flocs formed during the chemical pre- treatment, or undesirable chemicals (foam Floatation) are required to produce the air bubbles required for Floatation. Appendix B.4 (iii) illustrate the Performance of the Dispersed-Air Floatation
1.5.1.18 Aeration
Aeration is the process whereby water is brought into intimate contact with air. Aeration has a large number of uses in water treatment for the following purposes:
i.Increasing dissolved oxygen content in the water;
ii.Reducing tastes and odours caused by dissolved gases in the water, such as hydrogen sulphide, which are then released; and also to oxidise and remove organic matter;
iii.Decreasing carbon dioxide content of water and thereby reduce its corrosiveness and raise its pH value;
iv.Oxidizing iron and manganese from their soluble states to their insoluble states and thereby cause them to precipitate so that they may be removed by clarification and filtration processes;
v.Reduction of radon; and
vi.Removing certain volatile organic compounds.
Aeration is widely used for the treatment of groundwater having unacceptably high contents of dissolved iron and/ or manganese. The atmospheric oxygen brought into the water through aeration, reacts with the dissolved ferrous and manganous compounds, changing them into insoluble ferric and manganic oxide hydrates. The hydrates can then be removed by the subsequent processes of sedimentation and/or filtration.
Chemicals removed or oxidized by Aeration
a)Ammonia
b)Chlorine
c)Carbon dioxide
d)Hydrogen sulphide
e)Methane
f)Iron and Manganese
g)Volatile organic chemicals, such as benzene (found in gasoline), or trichloroethylene, dichloroethylene, and perchloroethylene (used in dry- cleaning or industrial processes)
Types of Aerators
Aerators fall into two categories:
- Falling water aerators
- Injection aerators
1.5.1.19 Falling Water Aerators
In the falling water aerators, water is dropped through air and in the second group air is introduced into the water as small bubbles. Falling water aerators can be divided into:
- Spray Aerators
- Multiple Tray Aerators
- Cascade Aerators
1.5.1.20 Spray Aerators
Water is sprayed through nozzles upward into the atmosphere and broken up into either a mist or droplets. Water is directed vertically or at a slight inclination to the vertical. The installation consists of trays and fixed nozzles on a pipe grid with necessary outlet arrangement.
(a)Design details of Spray Aerators
- Nozzles usually have diameters varying from 10 to 40 mm, spaced along the pipe at intervals of 0.5 to 1m or more. Special (patented) types of corrosion resistant nozzles and sometimes plain openings in pipes, serving as orifices are used.
- The pressure required at the nozzle head is usually 7 m of water but in practice, varies from 2 – 9 m and the discharge rating per nozzle varies form 30 - 600 l/min
- Aerator areas are usually 30 – 90 m2 per 1,000 m3/hr.
- The ‗Dresden‘ type of nozzles gives very good results in removing CO2 and in adding O2 but is poor for radon removal.
(b)Multiple Tray Aerators
These aerators consist of a series of trays with perforated bottoms. The trays are filled with coke, stone or ceramic balls, limestone, or other materials having a catalytic effect on iron removal. The primary purpose of the materials is providing additional surface contact area between the air and water. Through perforated pipes, the water is divided evenly over the upper tray, from which it trickles down, the droplets being dispersed and re-collected at each successive tray. Appendix B.5 (iii) illustrate the Multiple Tray Aerators.
(c)Design details of Multiple Tray Aerators
- 3-5 trays are normally used at the intervals of 0.3 - 0.7 m which means that the head needed is 1.5 – 3 m.
- The area required is 40 m2 per 1,000 m3/hr. These aerators have good CO2 removal and good 02 increases (3rd edition Design Manual, 2009).
- The design surface loading of a multiple tray aerator should be of the order of 70 m3/hour/m2 (Water supply Design Manual, Uganda (2013).
However, disadvantages of Multiple Tray Aerators are:
- risk of clogging
- difficult cleaning and breeding places for worms
(d)Cascade Aerators (Gravity Aerator)
The Cascade Aerators are the simplest type of free-fall aerators and will take large quantities of water in a comparatively small area and at low head. They are simple to keep clean and can be made of robust durable material such as reinforced concrete and are best in the open air. Turbulence is secured by allowing the water to pass through a series of steps or baffles (3rd edition Design Manual, 2009).
(e)Features of Cascade Aerator
a)A cascade aerator consists of a flight of 4 - 6 steps, each about 300 mm high, to produce turbulence and thus enhance the aeration efficiency, obstacles are often sat at the edge of each step as illustrated in Figure 7.25.
b)The design capacity of a cascade aerator should be of the order of
3
35 m /hour per meter of width.
c)Exposure time can be increased by increasing the number of steps which is normally between 3 - 10.
d)The fall in each step is usually between 0.15 - 0.6 m. The area required is about 40 m2 per 1,000 m3/hr.
e)The efficiency for raising 02 content is good and can reach 2.5 kg O2/kWh, but that for CO2 removal rarely better than 60 – 70 %, whilst radon reduction can exceed 50%.
f)The principle is to spread the water as much as possible and let it flow over obstructions to produce turbulence.
g)These are similar to tray aerators, but with a series of steps or platforms over which the water cascades. Obstacles may be placed on the edge of each step.
h)Cascades aerators generally take up more space than tray aerators, but the overall head loss is lower, and maintenance is minimal.
i)Where space permits, Cascade aerators are the preferred type of aerator.
Design details of Cascade Aerators
- Number of drops = 4-6
- Height of drops = 30-60 cm
- Overflow rate = 0.01 m3/s over meter width of step
- Height of aerator = 2-3 m
- Cascade area = 1.5-2.0 m2/m3/min of flow
1.5.1.20.1 Injection Aerators
These aerators have a good efficiency in raising O2 content but poor CO2 removal. They can be categorised as:
a)Bubble Aerators
b)Venturi Aerators
c)Brush Aerators
d)Inka Aerators
(a)Bubble Aerators
In these aerators, air is blown to the bottom of the tank through porous filters. Bubble aerators are often applied to existing treatment plants where no spare hydraulic head is available
Design details of Bubble Aerators
i.The depth of the tank is 3 - 4.5 m and the width about 2 times the depth.
ii. Detention time in the tank is 10 - 20 minutes.
iii. Air required is 40 – 120 m2 per 1,000 m3/hr, being 40% to 80% of water capacity.
iv. The air bubbles should be as small as possible but the clogging of the filters interfere with that aspect.
v.Good mixing in an aeration tank improves efficiency.
(b)Venturi Aerators
In venturi aerators, air is not blown into the water but is drawn in by a venturi. The 02 improvements is good but CO2 removal is poor.
(c)Brush Aerator
This type of aerator consists of a revolving drum, diameter about 0.5 m, submerged about 0.4m of the diameter, and rotating about 100 rpm. However, this type is not commonly used for water treatment.
(d)Inka Aerators
This aerator consists of a perforated stainless steel plate under which air is blown. Water flows over the plate. The air-water ratio is very high. It can be as high as 100:1. This amount of air causes heavy turbulence and so O2 raising efficiency and CO2 removal are good. Energy consumption is rather a high corresponding to a hydraulic head of 7m if air-water ratio is 100. A disadvantage is the clogging of the perforated plate.
1.5.2 Secondary Treatment
In this DCOM Manual, the following unit operations have been described in detail as secondary treatment. However, constructed wetlands are simply mentioned because they are described in detail in volume II of the manual.
- Coagulation
- Flocculation
- Clarification
- Filtration (SSF, RSF and other types)
- Reverse Osmosis
- Membrane Filtration (ultrafiltration (UF), Microfiltration (MF), nanofiltration (NF)
- Ion Exchange
- Adsorption
- Softening
1.5.2.1 Clarification
Clarification is a process of removing all kind of particles, sediments, oil, natural organic matter and colour from the water to make it clear. A clarification step is the first part of conventional treatment for water and wastewater treatment. It usually consists of physical and/or chemical treatment. Coagulation is normally followed by flocculation in a clarifier, which could be circular or rectangular in shape. After clarification water is then ready for filtration.
1.5.2.2 Coagulation
Coagulation is the process of adding a chemical (coagulant) to the raw water containing colloidal matter to form small gelatinous precipitated masses, which can readily settle out in sedimentation tanks within the normal range of surface loading. The coagulation stage occurs when a coagulant is added to the water to neutralise the charges on the colloidal particles in the raw water, thus bringing the particles closer together to allow a floc to begin to form. Rapid, high energy mixing (e.g. mechanical mixers, in-line static mixers, jet sparge mixing) is necessary to ensure the coagulant is fully mixed into the process flow to maximise its effectiveness. The coagulation process occurs very quickly, in a matter of fractions of a second. Poor mixing can result in a poorly developed floc. The most common coagulant in use in Tanzania is Aluminuim Sulphate (Alum), which at times is supplemented with coagulant aids.To determine the correct chemical dosage for aluminium sulphate solution and for water disinfection, jar testing is recommended.
1.5.2.3 Flocculation
The flocculation process, following coagulation, allows smaller particles formed during the rapid coagulation stage to agglomerate into larger particles to form settleable and/or filterable floc particles. After coagulant addition, the process water is mixed slowly for a defined flocculation period, commonly 10 - 30 minutes. However the optimum flocculation time will vary depending on the raw water quality and
downstream clarification process. Gentle mixing during this stage provides maximum particle contact for floc formation, whilst minimising turbulence and shear which may damage the flocs. Effectiveness of flocculation depends on the delay (or contact) time and mixing conditions prior to any flocculants being added, the rate of treatment, water temperature and the mixing conditions within the flocculation chamber. Flocculation takes place in a flocculator. There are two types of flocculators namely hydraulic and mechanical.
1.5.2.4 Filtration
Filtration is the process in which organisms, bacteria and particles of size less than 10-8 cm are removed. There are four main types;
- slow sand filters
- rapid (gravity) sand filters
- pressure filters
- upflow sand filters
Sand filters become clogged with floc after a period in use and they are then backwashed or pressure washed to remove the floc. This backwash water is usually run into settling tanks so that the floc can settle out and it is then disposed of as waste material. The supernatant water is sometimes run back into the treatment process, although this can bring some problems with it, or disposed off as a wastewater stream.
The criteria for designing filters are:
- Flow Rate
- Size of Media
- Depth of Media
- Type of Media
- Arrangement of gradation of Media
- Fluid characteristics
- Head loss
- Length of run
- Method of cleaning
A detailed description of filtration design considerations, criteria and steps have been given in the primary treatment section.
1.5.3 Tertiary Treatment
Tertiary treatment has considered the following unit operations
- Disinfection,
- Ozonation,
- Water softening and
- Water conditioning
The single most important requirement of drinking water is that it should be free from any micro-organisms that could transmit disease or illness to the consumer. Processes such as storage, sedimentation, coagulation and flocculation and rapid filtration reduce to varying degrees the bacterial content of water. However these processes cannot assure that the water they produce is bacteriologically safe, therefore disinfection is finally needed. Disinfection is carried out observing the following criteria:
- The nature and number of organisms to be destroyed
- The type and concentration of the disinfectant used
- The temperature of water to be disinfected
- The time of contact needed
- The nature of water to be disinfected
- The pH, acidity/alkalinity of the water
1.5.3.1 Disinfection Methods
There are two principal methods for disinfecting water; one is physical and the other chemical. Further details about disinfection methods are given in Appendix H.
1.5.3.2 Chlorinators
A chlorinator is a device designed for feeding chlorine in to a water supply. Its functions are:
- to regulate the flow of gas from the chlorine container at the desired rate of flow.
- to indicate the flow rate of gas feeding
- to provide means or properly mixing the gas either with an auxiliary supply of water' or with the main body of the liquid to be disinfected.
The usual fittings and parts of a chlorination system are:
- Chlorine cylinder or drum supplied with its own main valve and filled with liquid and gaseous chlorine, under pressure.
- Fusible plug, a safety device provided on all cylinders and containers designed to meet temperatures often between 700C to 750C
- Reducing valve / vacuum regulator to bring the pressure of the gas down to between 70 to 30 kPa so that the pressure is below atmospheric (approx 100 kPa). This should be located in the storage room so that any leakage in the dosing room is into the feed pipes rather than into the room itself.
- Pressure gauges one to indicate the cylinder pressure and the other the delivery pressure
- A measurement device consisting of an orifice to measure upstream or downstream pressure of gas with manometer containing liquid of carbon tetrachloride
- A ―desiccator valve‖ or non-return valve containing concentrated sulphuric acid or calcium chloride through which the chlorine must pass to free it from moisture so that any corrosive action of the moist chlorine on the fitting is prevented.
Design considerations for chlorinators
The following should be considered while design chlorinators;
- Access to storage and dosing rooms should separate and be from the open air and doors should always open outwards.
- External windows should be avoided where possible with artificial illumination being provided throughout.
- Both storage and dosing rooms should be provided with low level outlet venting fans that either come on automatically when the door is opened or are activated from outside the room so that any leakage is purged to the outside before entering such rooms.
- High-level fresh air inlets should be provided, especially to the storage room.
1.5.3.3 Ozonation
Ozone has been proved to be one of the most effective disinfectants and is widely used to inactivate pathogens in drinking water (Xu, 2002). Transferred ozone dose is the critical parameter for the design of wastewater disinfection by ozonation. The process should have an efficient filtration step to meet stringent standards. A properly designed ozonation process is able to deactivate viruses and bacteria that may be contained in wastewater.
1.5.4 Water softening
Softening is the process of removing the dissolved calcium and magnesium salts that cause hardness in water. The hardness or soap-consuming power of water is due to the presence of bicarbonates, carbonates, sulfates, chlorides, and nitrates of calcium and magnesium. The dissolved compounds have the following negative effects:
(i)Soap destroying or increased soap consumption in laundries,
(ii)Deposition of scale in boilers and engine jackets,
(iii)Corrosion and incrustation of pipelines, joints valves and plumbing fixtures; and
(iv)Serious difficulties and detrimental effects in the manufacturing processes, e.g. textile finishing, dyeing, canning, paper making, ice manufacturing, tanning etc.
When water is hard, it can clog pipes and soap will dissolve in it less easily. In industrial-scale water softening plants, the effluent flow from the re-generation process can precipitate scale that can interfere with sewage systems. Hard water leads to the build-up of limescale, which can foul plumbing, and promote galvanic corrosion. Water softening is the removal of calcium, magnesium, and certain other metal cations in hard water. It is achieved either by adding chemicals that form insoluble precipitates or by ion exchange. Water softening is usually achieved using lime softening or ion-exchange resins but is increasingly being accomplished using Nanofiltration or reverse osmosis membranes
Chemicals used for softening include calcium hydroxide (slaked lime) and sodium. The resulting soft water requires less soap for the same cleaning effort, as soap is not wasted bonding with calcium ions. Soft water also extends the lifetime of plumbing by reducing or eliminating scale build-up in pipes and fittings. Detailed information about hardness is provided in Appendix I
1.5.4.1 Methods of Softening
The most common means for removing water hardness (calcium and/or magnesium) and hence achieve softening are:
- Chemical precipitation;
- Ion-exchange resin; and
- Reverse Osmosis (RO)
(a) Softening By Chemical Precipitation
Chemical precipitation is among the most common methods used to soften
water. Chemicals used are lime (calcium hydroxide, Ca(OH)2) and soda ash
(sodium carbonate, Na2CO3). Softening by chemical precipitation is accomplished
by adding lime or lime and soda ash. Softening with these chemicals is used
particularly for water with high initial hardness greater than 500 mg/l and
suitable for water containing turbidity, colour, and iron salts because these have
a tendency to inactivate the ion exchange bed, by a coating on the granules.
Lime-soda softening cannot, however, reduce the hardness to values less than
40 mg/l and this should not be attempted.
Ion-exchange softening can produce zero-hardness water but such water should always be blended with water to leave a residual hardness of not less than 70 mg/l because apart from the risk of cardiovascular problems, very soft drinking water may be corrosive and result in feelings of sickness.
(i) Lime Treatment
Lime softening is the process in which lime is added to hard water to make it
softer. It has several advantages over the ion-exchange method but requires fulltime,
trained personnel to run the equipment. Addition of lime to hard water only
removes the carbonate hardness. Insoluble carbonates of calcium and magnesium
are precipitated out and removed in sedimentation tanks. (An overdose of lime
is usually used and the excess lime is neutralized by re-carbonation before
filtration). This treatment is good when the bulk of the hardness is due to calcium
and magnesium is insignificant. When the water contains more than 40 mg/l of
magnesium warranting its removal, excess lime treatment must be done.
(ii) Lime – Soda Treatment
Lime is used to remove chemicals that cause carbonate hardness, while Sodaash
is used to remove chemicals that cause non-carbonate hardness. In lime
treatment only the carbonate hardness is removed but by addition of soda, the
non-carbonate hardness is also removed, thus the removal of both carbonate
as well as non-carbonate hardness is possible in the lime-soda process. This
happens because in lime-soda ash softening process Ca2+ is removed from water
in the form of calcium carbonate, CaCO3 (s) and Mg2+ is removed in the form
of magnesium hydroxide, Mg(OH)2 (s). These precipitates are then removed by
conventional processes of coagulation/flocculation, sedimentation, and filtration.
Because precipitates are very slightly soluble, some hardness remains in the
water usually about 50 to 85 mg/l (as CaCO3). This hardness level is desirable to
prevent corrosion problems associated with water being too soft and having little
or no hardness. Precipitation of these salts is affected by the available Carbonate
species and pH of the system
- For calculating the theoretical amount of lime and soda required for softening, an analysis of the following constituents in the water is necessary:
- free carbon dioxide dissolved in the water bicarbonate (total alkalinity)
- total hardness
- total magnesium
Chemical requirement (mg/l) are computed by the sum of the following factors:
- Lime requirements as Ca(OH)2 (100% purity)
– 56/44 of concentration of CO2 (mg/l as CO2) – 56/24 of concentration of Mg (mg/l as Mg) – 56/100 of concentration of alkalinity (mg/l as CaCO3) Additional lime required for raising the pH to the range of 10 to 10.5 for precipitation of Mg(OH)2 is about 30 - 50 mg/l as CaO (Quick lime).
Soda requirements as Na2CO3 – 106/100 of difference between total hardness and bicarbonate alkalinity both expressed as CaCO3. – For neutralizing excess lime at 30 mg/l, additional soda required is (30/56) x 106 mg/l as Na2CO3. Plant conditions like temperature; time of detention and agitation influence the completeness of reactions and dosage of chemicals may have to be increased to provide for the inadequacies.
Alternatively, caustic soda can be used instead of lime. Liquid caustic soda should be used since it can be handled and fed easily. The amount calcium carbonate sludge formed in this case is theoretically half that formed by use of lime. However, using caustic soda is costlier than soda ash which is more expensive than lime.
(iii) Excess Lime Treatment
When water contains more than 40 mg/l of magnesium, excess lime treatment
has to be done since magnesium has to be removed as magnesium hydroxide
whose solubility decreases with increasing pH values. The water treated thus is
highly caustic and must be neutralised after precipitation either by re-carbonation
or by split treatment. In split treatment, the total flow is divided into two parts,
one part being treated with excess lime and the settled effluent then mixed with
un-softened water. The final residual hardness in the water will depend on the
percentage flow by-passed and the levels of or hardness in both the portions
(treated and by passed).
(iv) Hot Lime-Soda Treatment
This process is used for boiler feed water treatment. It is similar to the cold
process already discussed except that the raw water is heated to about 95° -
100°C before being taken’ to the reaction tank. Reactions take place rapidly due
to decreased viscosity hastening the settling of the precipitates. A greater degree
of softening is accomplished than that in the conventional cold processes.
(v) Re-carbonation
After lime and/or soda ash treatment is applied, the treated water will generally
have a pH greater than 10. In addition, after softening, water becomes
supersaturated with calcium carbonate. If this water is allowed to enter the
distribution system in this state, the low pH would cause corrosion of pipes and
the excess calcium carbonate would precipitate out, causing scale. So, the water
must be re-carbonated, which is the process of stabilizing the water by lowering
its pH and precipitating out excess lime and calcium carbonate.
Therefore, the goal of re-carbonation is to produce stable water. Stable water has
a calcium carbonate level, which will neither tend to precipitate out of the water
(causing scale) nor dissolve into the water (causing corrosion). This goal is usually
achieved by pumping CO2 into the water. Enough CO2 is added to reduce the pH
of the water to less than 8.7. When CO2 is added, the excess lime will react with
CO2 producing CaCO3 (s). Recarbonation also lowers the water pH.
(b)Water Softening through Ion-Exchange
The ion-exchange process is the reversible interchange of ions between a solid
ion exchange medium and a solution and is used extensively in industrial water/
softening. The hardness producing ions preferentially replace the cations in the
exchangers and hence this process is also known as base or cation exchange
softening.
The ion exchange works on the hydrogen or sodium cycle. The hydrogen ions are released into the water in the former case and the sodium ions in the latter. There is only a temporary change in the structure of the exchange material. The exchange material can be re-generated using acid and sodium chloride respectively.
In general, ion exchange materials used in water softening, also called zeolites, are hydrated silicates of sodium and aluminum. There are inorganic and organic zeolites:
(i) Inorganic Zeolites
Natural inorganic zeolite is available as ‘green sand’ while the synthetic or gel
type is obtained through the reaction of either sodium aluminates or aluminium
and is graded to suitable sizes by the reaction of either sodium aluminate or
aluminium sulphate with sodium silicate which, after drying, is graded to suitable
sizes by screening. For regeneration, 3.5 to 7 kg of salt is required for every
kilogram of water hardness removed.
(ii) Organic Zeolites
These consist of carbonaceous materials and sulphonated stayrone type resins
which have excellent cation exchange properties, requiring for regeneration, 2-4
kg salt for every kilogram of hardness removed. These are resistant to attack by
acid solutions and hence can be regenerated by acid. They can be used for waters
with a wide pH range, whilst the loss due to attrition is negligible compared to the
synthetic inorganic zeolites.
(iii) Raw water characteristics
Raw water to be treated by ion exchange should be relatively free from turbidity
otherwise the exchange material gets a coating that affects the exchange
capacity of the bed. The desirability of using filters prior to zeolite beds or resorting
to more frequent regeneration would depend upon the level of turbidity. Metal
ions like iron and manganese, if present, are likely to be oxidized and can coat
zeolites, thus deteriorating the exchange capacity steadily since the regeneration
cannot remove the coats.
Oxidizing chemicals like chlorine and carbon dioxide as well as low pH in the water will have a tendency to attack the exchange materials particularly the inorganic type, the effect being more pronounced on the synthetic inorganic zeolites. Waters low in silica inorganic zeolites, are to be avoided in boiler feed water. The organic zeolites, operating on a brine regeneration cycle do not add any silica to the water and consequently are ideally suited for boiler feed water.
Caution
The ion exchange process is both costly and delicate and should not be adopted
without advice from a competent authority. In case the need arises for using this
type of process for water softening then the details of the process design should
be obtained from a standard textbook or plant manufacturer.
1.5.5 Defluoridation of Water
1.5.5.1 Fluorides
Fluoride is the ionic form of fluorine. Fluorides are organic and inorganic compounds containing the element fluorine. As a halogen, fluorine forms a monovalent ion (−1 charge). Fluoride forms a binary compound with another element or radical. Examples of fluoride compounds include hydrofluoric acid (HF), sodium fluoride (NaF) and calcium fluoride (CaF2), and uranium hexafluoride (UF6).
Fluoride compounds, usually calcium fluoride, are naturally found, usually in low concentrations in water. However, water from underground sources can have higher levels of fluoride to the level that it becomes a health hazard.
Excessive fluorides in drinking water may cause mottling of teeth or dental fluorosis, a condition resulting in the coloration of the tooth enamel, with chipping of the teeth in severe cases, particularly in children. With even higher levels of fluorides, there are cases of fluorosis of the bony structure.
The chief sources of fluorides in nature are:
- fluorapatite (phosphate rock)
- fluorspar
- cryolite and
- igneous rocks containing fluorosilicates
A designer, in deciding on whether or not to include de-fluoridation in a water supply scheme should, therefore, consider both the number of potential consumers, alternative sources, the financial consequences both in capital and running and whether or not there is a possibility to dilute the water containing the fluoride as a means of reducing the concentration. Defluoridation technology opted by the designer should ensure that the product (treated water) meets relevant Tanzania Standards and other standards/guideline which may have been adopted by the country.
1.5.5.2 Defluoridation
Defluoridation is necessary when the fluoride concentration is higher than the acceptable limits. The following methods may be considered for attaining defluoridised water standards.
- Desalination
- Additive methods
- Absorption methods
Desalination
Desalination effectively removes all dissolved impurities from water. This can be
accomplished in one of several ways, by freezing, by distillation, by electrolysis or
by reverse osmosis. The cost of desalination is high although the costs of reverse
osmosis have fallen considerably in recent years. Nevertheless, this method is
more appropriate to deal with brackish or sea water although it should not be
entirely ruled out where there a few, if any, alternative sources and there is a
good supply of electricity.
Additive method
In this method, one or more chemicals are added to water. The fluoride is then
absorbed and both the additive and the fluoride are consequently removed by
using conventional treatment processes such as sedimentation and filtration. A
wide variety of materials have been tried including lime, magnesium sulphate,
magnesium oxide, calcium phosphate, aluminium sulphate, various natural
earths, bauxite, sodium silicate and sodium aluminate.
Excessive lime treatment for water softening affects the removal of fluoride due to its absorption by the magnesium hydroxide floc. However, sizeable fluoride removal is possible only when magnesium is present in large quantities which may not always be the case and magnesium may have to be supplemented in the form of salts.
The initial cost and cost of chemicals is very high and the resultant sludge is environmentally difficult to dispose of.
Absorption methods Absorption methods employ a bed or filter of generally insoluble material through which the water is allowed to percolate periodically. As it becomes saturated, with the fluoride ‘removed, the absorptive media is either replaced or appropriately regenerated.
(a) Materials used have included charred bone, activated alumina, activated carbon, tricalcium phosphate, natural and synthetic ion-exchange materials and aluminum sulfate. (b) Studies elsewhere have revealed that activated carbon has a good capacity to remove fluoride where the concentration is less than 10 mg/l and the water is low in salinity. (c) An activated carbon for fluoride removal has been developed in India by carbonizing paddy husk or sawdust, digesting under pressure with alkali, and quenching it in a 2% alum solution. The spent material can be regenerated by soaking it in a 2% alum solution. (d) Also a granular ion exchange material, ‘Defluoron 2’, which is sulfonated coal operating on the aluminum cycle has been developed in India. (e) The water treatment specialist M/S Degrémont recommended the activated alumina in the case of the Arusha urban water supply project.
However, defluoridation must be regarded as a sophisticated process and to determine suitability and quantity of chemical, pilot plant trials should be conducted first.
1.5.6 Water Conditioning
Water conditioning entails ensuring that at the end of the treatment process but just before the water is pumped or gravitated into the clear water reservoir, storage tanks or the distribution network, the treated water should be neither precipitative nor corrosive. Hence this may require a pH correction in either direction depending on the outcome of the daily laboratory analysis results. Moreover, if there will be a need to increase the pH, lime will have to be prepared as a slurry for the sake of controlling the dosage and hence dosed at the point of dosing the coagulants and other chemical additives. In case the pH of water has to be increased, a dose of an acid would suffice. Care has to be taken against introducing major shifts of pH due to the risk of making it limiting as for example when Ferrous chemicals are used in coagulation of water.
1.5.7 Management of Water Treatment Sludge
The conventional water treatment plant involves the process of coagulation, flocculation, sedimentation, filtration and disinfection. Large volumes of water treatment sludge (WTS) or residues thereof are generated during the processing of raw water to make it fit for drinking purposes. A typical water treatment plant produces about 100,000 ton/year of sludge (Bourgeois et al., 2004). Experiences elsewhere have shown that, due to the lack of sludge management strategies, most of the WTPs discharge their filter backwash water and sludge into nearby drains which ultimately meet the water source. Aluminium salts (e.g. Al2(SO4)3.18H2O) or Iron salts (e.g. FeCl3.6H2O, FeCl2, FeSO4.7H2O) are commonly used as coagulants (Sales et al., 2011). These salts get hydrolysed in water to form their respective hydroxide precipitates. Colloidal and suspended impurities such as sand, silt, clay, humic particles present in the crude water are removed by charge neutralization, sweep floc mechanism and adsorption onto hydroxide precipitates (Trinh and Kang, 2011). The hydroxide precipitate along with sand, silt, clay and humic particles removed from the raw water mainly constitute the solids present in the sludge. The moisture content of the wet sludge is generally above 80 wt% (Tantawy et al., 2015). In general, this sludge is discharged directly into nearby water bodies or dumped in the landfills after dewatering.
1.5.8 Treatment of Water Treatment Sludge
1.5.8.1 Sludge Thickening
Sludge thickening is defined as the removal of water from the sludge with the aim of substantially reducing sludge volume. For example if sludge with 0.8% dry solids (DS) can be thickened to 4% DS; a fivefold decrease in sludge volume is achieved. The objective of sludge thickening is to produce a sludge that is as thick as possible which can be pumped without difficulty and includes a relatively solid free liquid supernatant.
(a) Gravity Thickening
(i) Description of Unit
Gravity sludge thickening is the method commonly adopted. The slope of the
bottom of the gravity thickeners should be carefully selected in order to facilitate
the flow of thickened sludge towards the center/collection pit. Gravity thickeners,
usually circular in shape and provided with pickets or rakes to improve the dewatering
of sludge. A dry solids content of 2-2.5% can be expected from gravity thickening.
The gravity thickening design is similar to a clarifier. Thickeners are usually circular shaped;
and the feed is carried out through a pipe to a central hood serving
as distribution and still area, with a height that has no effect on compaction or
compression bottom area. Except for small thickeners, static, and with hopper
floor, these units have a system of very strong bottom scrapers, which carry the
sludge to a central tank and on which pickets are installed. These vertical bars, that
move smoothly, enhance mass homogeneity and create preferential channels
enabling the disposal of interstitial water and occluded gases generated by
fermentation phenomena and facilitating the thickening. The supernatant liquid
is collected by a perimeter weir and sent to the plant head or primary treatment.
(ii) Design considerations and procedures Hydraulic loading rate and solids load are the main design parameters. The hydraulic loading rate is based on the real flow through the unit, that is, which goes by the perimetral discharge weir(s) (outflow).
Where,
CS = solids load (kg SS/m2/h)
Q = sludge flow to the thickening unit (m3/h)
X = solids concentration (mg/L)
A = horizontal thickener surface (m2)
Sludge Dewatering
The alternatives for sludge dewatering systems are described below. Guidance
for the selection of an appropriate system is given in Table 7.5.
Sludge Drying Beds
Sludge drying beds are most favoured when lands are available in close proximity
to the water treatment works. Areas where strong sunlight is available with
average annual rainfall lower than 2,200 mm are appropriate. The filtrate of
the sludge drying beds can be directed under gravity to sludge regulation tank
for subsequent thickening and should not be discharged to the environment.
However in areas having higher rainfall (average annual rainfall between 3000 to
6000 mm) in order to achieve higher dry solid contents solar sludge drying beds
having a roof cover of UV protected polythene can be utilized.
Sludge Lagoons
Lagoons may be the cheapest method of sludge dewatering but large land
area are required compared to mechanical dewatering techniques. However,
compared to conventional sludge drying beds, lagoons require considerably
lesser land. Lagoons can be lined or unlined or can be provided with under
drain arrangement for better dewatering requirements. Unlined earthen sludge
lagoons are more effective in dealing with large volumes of sludge from higher
capacity water treatment plants. However due consideration should be given
to the following during planning of the water treatment plant layout to locate
sludge lagoons:
- Fluctuation of groundwater table (seasonal high groundwater table should be- sufficiently below the bottom of the lagoon preferable below 2.5 m),
- Underlying soil characteristics should be investigated to see its suitability,soil- percolation rate between 25 to 150 mm/hr being preferred,
- Annual precipitation preferably to be below 3,000 mm,
- Access ramps to be provided for dumper/tractor/mini loader to collect driedsludge from the lagoon and final disposal.
1.5.8.2 Mechanical Sludge Dewatering
Important operational parameters to be considered in evaluation of these systems are;
- energy consumption,
- required polymer dosage and
- separation efficiency
Further, mechanical dewatering systems such as;
- Belt Filter Presses,
- Filter Presses and
- Centrifuges should operate continuously as far as possible in order to reduce usage of treated water for the cleaning operation required at the end of each operation cycle and to optimize utilization of equipment.
These equipment require
- high skilled maintenance staff
- suitable polyelectrolyte with dosing arrangement
- electrical power for the operation of the equipment
Therefore, the process is usually attractive only in large sludge dewatering facilities with incoming flow > 0.3 m3/s (25,920 m3/day). As such, correct assessment of sludge generation and selection of the capacity of each unit is very important. After mechanical dewatering, the sludge is generally directed through a conveyer system into a skip or a hopper. The filtrate from mechanical dewatering facility can be directed back to the sludge regulation tank. Most mechanical dewatering equipment can achieve 15-20% DS content but the actual performance needs to be verified from the manufacturers. Mechanical sludge dewatering is only recommended for:
- major water treatment plants that generate large quantities of sludge,
- treatment plants that do not have adequate land or areas that experience average annual rainfall in excess of 3,000 mm.
1.5.8.3 Backwash Water Recovery
Backwash recovery aims at utilizing water resources to maximum potential, to minimize energy consumption and thereby optimize production costs. The backwash recovery process should not cause any adverse impacts on the treated water quality. The possible health implications could be trace amounts of heavy metals that may be present in raw water or in water treatment chemicals as impurities, pathogenic microorganisms, algal toxins/THM which may be produced during physical/chemical processes of the treatment works or present in raw water. In this context, reuse of thickener supernatant is not recommended as about 95% of the contaminants in raw water are removed from the sedimentation process and hence may contain pathogenic organisms such as Cryptosporidium and Giardia Lamblia which are resistant to chlorination. It is advantageous to reuse the backwash water in order to conserve energy by minimizing the utilization of low lift pumps and to recover 2-5% of water. Backwash water recovery has many advantages. However, the introduction of backwash water recovery needs careful evaluation of the raw water quality, the proposed treatment process and the cost-benefits.
Therefore, the recommended unit processes for backwash water recovery to be incorporated in the sludge treatment stream are as follows:
- Backwashed water is first directed to backwash recovery tank (minimum two tanksü each having capacity at least to hold two backwashes) where it is allowed to settle for a selected time,
- After allowing for sedimentation, supernatant is gravity fed to backwash recirculation tank which is constructed with common wall to the backwash recovery tank to minimize construction cost,
- The gravity feeding system should comprise pontoon attachment at the the surface of the inlet pipe (which needs to be covered with suitable mesh to prevent escaping of floating debris) and the bottom end supported by a hinged bend in order to facilitate rotation of the pontoon attached inlet pipe as water level drops up to the sludge thickening zone of the backwash recovery tank.
1.5.8.4 Waste from Slow Sand Filters
During the scraping operation of the biological layer called “schmutzdecke” of the slow sand filters, the removed sand can be washed using “hydro cyclone” and reused when “re-sanding” of the slow sand filters is required. The dirty water resulting from the hydro cyclone needs to be treated appropriately in line with the disposal standards. A sludge thickener can be used if the number of filters is higher and continuous type of treatment is needed as in larger scale water treatment plants. If the treatment plant is of small scale, it may be sufficient to have a roughing filter together with natural or constructed wetland to polish further to meet the discharge standards as this type of system may be suitable for intermittent operation as generally slow sand filters need to be cleaned once in two to three months depending on the raw water quality.
1.5.8.5 Disposal of Sludge
The dried sludge resulting from different dewatering methods discussed above
should be disposed in compliance to environmental regulations. The options
available for disposal of sludge are:
(a) Land Disposal
(i) Forest
(ii) Land Reclamation
(iii) Landfill
(b) Incineration
(c) Melting
(d) Brick and roof tile construction (after mixing with other constituents to
obtain the desired consistency)
REFERENCES
Eliakimu, N., R. Machunda and K. Njau (2018). “Water quality in earthen dams and potential health impacts: case of Nadosoito Dam, Tanzania.” Water Practice and Technology13(3): 712-723.
Jordan K. Lanfair, Stephen T. Schroth, Archis Ambulkar (2020). Desalination. Retrieved from: https://www.britannica.com/technology/prechlorination
Huisman (1974).https://sswm.info/sswm-university-course/module-6-disaster-situationsplanning-and-preparedness/further-resources-0/slow-sand-filtration)
Mbwette, T.S.A and Wegelin 1984. Field Experience with HRF-SSF Systems in Treatment of Turbid Surface Waters in Tanzania. Proc. IWSA Congress, Monathis, Tunisia pp 10-12 (SS6)
https://www.slideshare.net/gowrivprabhu/water-treatmentwater-treatment
Previous Page: Chapter_Six:_Pumping_Systems << >> Next Page: Chapter_Eight:_Treatment_of_Waters_With_Special_Contaminants