Difference between revisions of "Chapter Twelve: Water Treatment"
| (18 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| − | = | + | <div style="text-align:justify"> |
| + | |||
| + | |||
| + | = Chapter Twelve: Water Treatment = | ||
The principal objective of water treatment in water supply industry is to produce water that is fit for domestic use from a raw water source throughout the water supply system to the consumers. The raw water available from sources particularly surface water sources is normally not suitable for drinking purposes. Thus, raw water needs treatment to produce safe and potable drinking water. Some of the common treatment processes of the conventional treatment facilities include the following:<br> | The principal objective of water treatment in water supply industry is to produce water that is fit for domestic use from a raw water source throughout the water supply system to the consumers. The raw water available from sources particularly surface water sources is normally not suitable for drinking purposes. Thus, raw water needs treatment to produce safe and potable drinking water. Some of the common treatment processes of the conventional treatment facilities include the following:<br> | ||
| Line 191: | Line 194: | ||
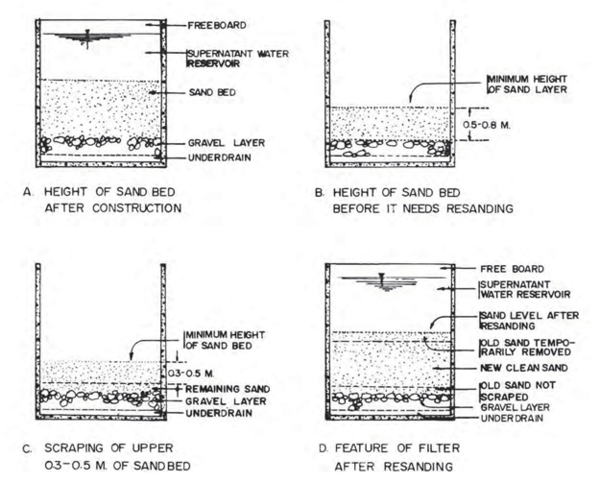
Re-sanding becomes necessary when the depth of the sand bed drops to its minimum designed level (usually about 0.5 – 0.8 m above the supporting gravel, depending on the grain size of the filter sand/medium). This depth is usually indicated by a marker (such as a concrete block or a step in the filter box wall) set in the structure during the original construction to serve as an indication that this level has been reached and that sanding has become due. After scraping, add new clean sand up to a level shown in Figure 12.1 and place back the old sand that was scraped off the top. The old sand will reduce the number of days needed for ripening the filter. | Re-sanding becomes necessary when the depth of the sand bed drops to its minimum designed level (usually about 0.5 – 0.8 m above the supporting gravel, depending on the grain size of the filter sand/medium). This depth is usually indicated by a marker (such as a concrete block or a step in the filter box wall) set in the structure during the original construction to serve as an indication that this level has been reached and that sanding has become due. After scraping, add new clean sand up to a level shown in Figure 12.1 and place back the old sand that was scraped off the top. The old sand will reduce the number of days needed for ripening the filter. | ||
| − | [[File:Figure_12Details_of_Cleaning_and_Re-sanding_of_the_SSF.PNG|600px|Link=Chapter_Twelve:_Water_Treatment]] <br> | + | [[File:Figure_12Details_of_Cleaning_and_Re-sanding_of_the_SSF.PNG|600px|center|Link=Chapter_Twelve:_Water_Treatment]] <br> |
Figure 12. 1: Details of Cleaning and Re-sanding of the SSF | Figure 12. 1: Details of Cleaning and Re-sanding of the SSF | ||
(Source: World Bank, 2012) | (Source: World Bank, 2012) | ||
| Line 250: | Line 253: | ||
==== Maintenance of Aeration Equipment ==== | ==== Maintenance of Aeration Equipment ==== | ||
| + | |||
Proper maintenance of aerators is another important area in water treatment activities. Maintenance is on the following elements;<br> | Proper maintenance of aerators is another important area in water treatment activities. Maintenance is on the following elements;<br> | ||
| + | |||
'''1. Waterfall Aerators'''<br> | '''1. Waterfall Aerators'''<br> | ||
The recommended maintenance procedures for waterfall-type aerators (cascade or step, and tray or splash pan) is as follows:<br> | The recommended maintenance procedures for waterfall-type aerators (cascade or step, and tray or splash pan) is as follows:<br> | ||
| Line 285: | Line 290: | ||
a) [http://Source:http://constructionmanuals.tpub.com/14265/css/Maintenance-of-Aeration-Equipment-294.htm Source:http://constructionmanuals.tpub.com/14265/css/Maintenance-of-Aeration-Equipment-294.htm] <br> | a) [http://Source:http://constructionmanuals.tpub.com/14265/css/Maintenance-of-Aeration-Equipment-294.htm Source:http://constructionmanuals.tpub.com/14265/css/Maintenance-of-Aeration-Equipment-294.htm] <br> | ||
b) https://cdn2.hubspot.net/hubfs/541513/Brochures/Brochure-Aerators.pdf | b) https://cdn2.hubspot.net/hubfs/541513/Brochures/Brochure-Aerators.pdf | ||
| − | |||
== Secondary Treatment == | == Secondary Treatment == | ||
| Line 293: | Line 297: | ||
Clarification is a process of removing all kind of particles, sediments, oil, natural organic matter and colour from the water to make it clear. A clarification step is the first part of conventional treatment for water and wastewater treatment. It usually consists of physical and/or chemical treatment. Coagulation is normally followed by flocculation in a clarifier, which could be circular or rectangular in shape. After clarification water is then ready for filtration. | Clarification is a process of removing all kind of particles, sediments, oil, natural organic matter and colour from the water to make it clear. A clarification step is the first part of conventional treatment for water and wastewater treatment. It usually consists of physical and/or chemical treatment. Coagulation is normally followed by flocculation in a clarifier, which could be circular or rectangular in shape. After clarification water is then ready for filtration. | ||
| − | + | ===Coagulation and Flocculation=== | |
The term coagulation and flocculation are often used to describe the process of removal of turbidity caused by fine suspension, colloids and organic colours, i.e. non-settle able particles from water. | The term coagulation and flocculation are often used to describe the process of removal of turbidity caused by fine suspension, colloids and organic colours, i.e. non-settle able particles from water. | ||
| − | |||
| − | (a) Chemical Coagulants Commonly Used in Treatment Process | + | ===== Coagulation ===== |
| + | |||
| + | (a) '''Chemical Coagulants Commonly Used in Treatment Process''' | ||
| + | |||
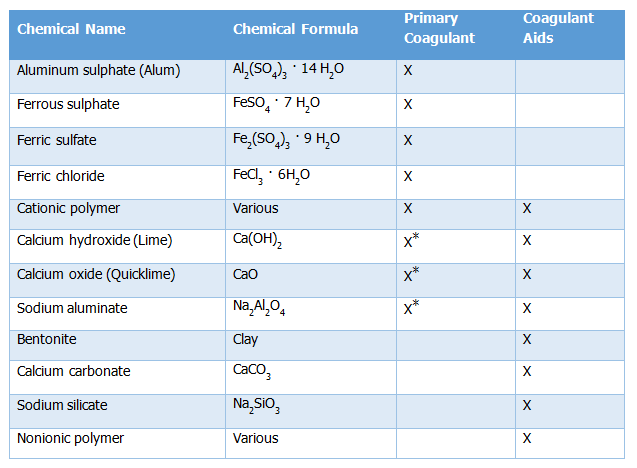
Coagulant chemicals are in two main types including primary coagulants and coagulant aids. Primary coagulants neutralize the electrical charges of particles in the water which causes the particles to clump together well as coagulant aids add density to slow-settling flocs and add toughness to the flocs so that they do not break up during the mixing and settling processes. Primary coagulants are always used in the coagulation/flocculation process while coagulant aids, in contrast, are not always required and are generally used to reduce flocculation time. | Coagulant chemicals are in two main types including primary coagulants and coagulant aids. Primary coagulants neutralize the electrical charges of particles in the water which causes the particles to clump together well as coagulant aids add density to slow-settling flocs and add toughness to the flocs so that they do not break up during the mixing and settling processes. Primary coagulants are always used in the coagulation/flocculation process while coagulant aids, in contrast, are not always required and are generally used to reduce flocculation time. | ||
| Line 307: | Line 313: | ||
Table 12. 1: Types of Coagulants/Aids | Table 12. 1: Types of Coagulants/Aids | ||
| − | + | ||
| − | + | [[File:12.PNG|700px|center]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
*Used as a primary coagulant only in water softening processes. | *Used as a primary coagulant only in water softening processes. | ||
(Source: Belmont Water Treatment Association as cited in the Water Supply Design Manual, Uganda, 2013). | (Source: Belmont Water Treatment Association as cited in the Water Supply Design Manual, Uganda, 2013). | ||
| − | (b) Tips for Selection of Coagulant | + | |
| + | |||
| + | (b) '''Tips for Selection of Coagulant''' | ||
| + | |||
Coagulation is a physical and chemical reactions occurring between the alkalinity of the water and the coagulant added to the water, which results in the formation of insoluble flocs. The most important consideration is the selection of the proper type and amount of coagulant chemical to be added to raw water. Over-dosing as well as under-dosing of coagulants may lead to reduced solids removal efficiency. This condition may be corrected by carefully performed Jar tests and verifying process performance after making any change in the process of the coagulation process. | Coagulation is a physical and chemical reactions occurring between the alkalinity of the water and the coagulant added to the water, which results in the formation of insoluble flocs. The most important consideration is the selection of the proper type and amount of coagulant chemical to be added to raw water. Over-dosing as well as under-dosing of coagulants may lead to reduced solids removal efficiency. This condition may be corrected by carefully performed Jar tests and verifying process performance after making any change in the process of the coagulation process. | ||
| − | (c) Aluminium Sulphate Coagulant | + | (c) '''Aluminium Sulphate Coagulant''' |
| + | |||
Aluminium sulphate is a chemical compound with the formula Al2(SO4)3. Aluminium sulphate is mainly used as a flocculating agent in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing. It is recommended to be used as the coagulant of choice in Tanzania | Aluminium sulphate is a chemical compound with the formula Al2(SO4)3. Aluminium sulphate is mainly used as a flocculating agent in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing. It is recommended to be used as the coagulant of choice in Tanzania | ||
| − | (d) Use of Aluminium Sulphate | + | (d) '''Use of Aluminium Sulphate''' |
| + | |||
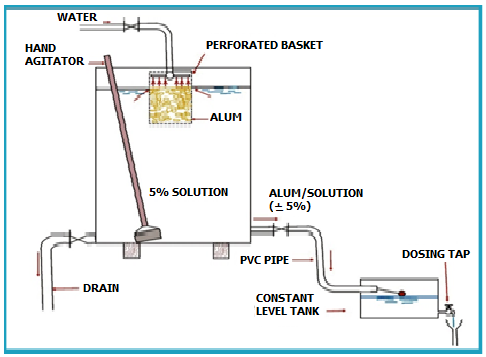
Two solution tanks, one for mixing and the other for dosing, between them holding 48 hours of supply, should be provided. The solution strength should be in the range of 5-10%.The solution tanks could be equipped with hand agitators as shown in Figure 12.2. | Two solution tanks, one for mixing and the other for dosing, between them holding 48 hours of supply, should be provided. The solution strength should be in the range of 5-10%.The solution tanks could be equipped with hand agitators as shown in Figure 12.2. | ||
| − | + | [[File:Figure12.PNG|500px|center]] | |
Figure 12. 2: Dosing Arrangement for Alum | Figure 12. 2: Dosing Arrangement for Alum | ||
| + | |||
If the alkalinity of the raw water is low, the pH can be appropriately adjusted by adding soda ash in the correct proportions, as determined after carrying out laboratory experiments called “jar tests”. The strength of the soda ash solution required is usually in the range of 1-10%.The solution tanks for soda ash should also hold a total of 48 hours of supply. The chemical solutions should be fed into the raw water by means of gravity dossers, floating balls or other similar simple devices. Dosing pumps should be used only in exceptional cases. | If the alkalinity of the raw water is low, the pH can be appropriately adjusted by adding soda ash in the correct proportions, as determined after carrying out laboratory experiments called “jar tests”. The strength of the soda ash solution required is usually in the range of 1-10%.The solution tanks for soda ash should also hold a total of 48 hours of supply. The chemical solutions should be fed into the raw water by means of gravity dossers, floating balls or other similar simple devices. Dosing pumps should be used only in exceptional cases. | ||
| − | (e) The Jar Test | + | (e) '''The Jar Test''' |
| + | |||
To determine the correct chemical dosage for aluminium sulphate solution and for water disinfection, jar testing is recommended (normally Jar test experiments are done in the Laboratory). Jar testing entails adjusting the amount of treatment chemicals and the sequence in which they are added to samples of raw water held in jars or beakers. The sample is then stirred so that the formation, development, and settlement of floc can be watched just as it would be in the full scale treatment plant. Jar testing should be done seasonally (temperature), monthly, weekly, daily, or whenever a chemical is being changed, or new pumps, rapid mix motors, new floc motors, or new chemical feeders are installed. There is no set requirement for how often jar testing should be conducted, but the more it is done the better the plant will operate. Optimization is the key to running he plant more efficiently. | To determine the correct chemical dosage for aluminium sulphate solution and for water disinfection, jar testing is recommended (normally Jar test experiments are done in the Laboratory). Jar testing entails adjusting the amount of treatment chemicals and the sequence in which they are added to samples of raw water held in jars or beakers. The sample is then stirred so that the formation, development, and settlement of floc can be watched just as it would be in the full scale treatment plant. Jar testing should be done seasonally (temperature), monthly, weekly, daily, or whenever a chemical is being changed, or new pumps, rapid mix motors, new floc motors, or new chemical feeders are installed. There is no set requirement for how often jar testing should be conducted, but the more it is done the better the plant will operate. Optimization is the key to running he plant more efficiently. | ||
| − | (f) Dosing of the coagulant at a spot of maximum turbulence | + | (f) '''Dosing of the coagulant at a spot of maximum turbulence''' |
Rapid mix of coagulant at a spot of maximum turbulence, followed by tapered flocculation in three compartmentalized units allows a maximum of mixing(reduced short circuiting), followed by a period of agglomeration intended to build larger fast settling flocs. | Rapid mix of coagulant at a spot of maximum turbulence, followed by tapered flocculation in three compartmentalized units allows a maximum of mixing(reduced short circuiting), followed by a period of agglomeration intended to build larger fast settling flocs. | ||
| − | (g) Mixing | + | (g) '''Mixing''' |
The mixing is the process to mix all the coagulant in water rapidly and instantaneously especially in waters with high alkalinity so as to achieve complete homogenization of a coagulant in the water to be treated. Mixing of the coagulant can be satisfactorily accomplished in a special coagulant tank with mixing devices or in the influent channel or a pipeline to the flocculation basin with high flow velocity which produces necessary turbulence. | The mixing is the process to mix all the coagulant in water rapidly and instantaneously especially in waters with high alkalinity so as to achieve complete homogenization of a coagulant in the water to be treated. Mixing of the coagulant can be satisfactorily accomplished in a special coagulant tank with mixing devices or in the influent channel or a pipeline to the flocculation basin with high flow velocity which produces necessary turbulence. | ||
To accomplish the mixing, following methods can be used: | To accomplish the mixing, following methods can be used: | ||
| + | |||
(i) Hydraulic mixing, | (i) Hydraulic mixing, | ||
| + | |||
(ii) Mechanical mixing, | (ii) Mechanical mixing, | ||
| + | |||
(iii) Diffusers and grid system, | (iii) Diffusers and grid system, | ||
| + | |||
(iv) Pump-blenders. | (iv) Pump-blenders. | ||
| − | (h) Storage of Aluminium Sulphate | + | |
| + | (h) '''Storage of Aluminium Sulphate''' | ||
| + | |||
Aluminium sulphate should be stored in a secured, cool, dry, well-ventilated area, removed from oxidising agents, alkalis, most metals, heat or ignition sources and foodstuffs. Ensure containers are adequately labelled, protected from physical damage and sealed when not in use. Check regularly for leaks or spills (if in a solution form). Large storage areas should have appropriate fire protection and ventilation systems. | Aluminium sulphate should be stored in a secured, cool, dry, well-ventilated area, removed from oxidising agents, alkalis, most metals, heat or ignition sources and foodstuffs. Ensure containers are adequately labelled, protected from physical damage and sealed when not in use. Check regularly for leaks or spills (if in a solution form). Large storage areas should have appropriate fire protection and ventilation systems. | ||
| − | (i) Health Hazards and Disposal of Waste Solution and Sludge | + | (i) '''Health Hazards and Disposal of Waste Solution and Sludge''' |
| + | |||
Aluminium sulphate is categorised as a slightly corrosive, irritant and hazardous substance. This product has the potential to cause adverse health effects with over long exposure time. Use safe work practices to avoid eye or skin contact and inhalation. It may hydrolyse (with addition of water) to sulphuric acid, a strong tissue irritant. If released to water environment; aluminium salts will slowly be precipitated as aluminium hydroxide. This may lower the pH of receiving waters with toxic effects to aquatic organisms. It is not expected to bio- accumulate. Water plants may experience chronic toxicity at around 25 ppm. Before disposal, neutralise the solution with lime, weak alkali or similar. For small amounts, absorb with sand or similar and dispose of to an approved landfill site | Aluminium sulphate is categorised as a slightly corrosive, irritant and hazardous substance. This product has the potential to cause adverse health effects with over long exposure time. Use safe work practices to avoid eye or skin contact and inhalation. It may hydrolyse (with addition of water) to sulphuric acid, a strong tissue irritant. If released to water environment; aluminium salts will slowly be precipitated as aluminium hydroxide. This may lower the pH of receiving waters with toxic effects to aquatic organisms. It is not expected to bio- accumulate. Water plants may experience chronic toxicity at around 25 ppm. Before disposal, neutralise the solution with lime, weak alkali or similar. For small amounts, absorb with sand or similar and dispose of to an approved landfill site | ||
| − | + | ===== Flocculation ===== | |
| + | |||
| + | (a) '''Flocculation Basin– Operation''' | ||
| − | |||
The objective of a flocculation basin is to produce a settled water of low turbidity which in turn leads to reasonably longer service period of filter plant. | The objective of a flocculation basin is to produce a settled water of low turbidity which in turn leads to reasonably longer service period of filter plant. | ||
| − | (b) Clari-flocculator | + | (b) '''Clari-flocculator''' |
| + | |||
The flocculators may be circular, square or rectangular. The best flocculation is usually achieved in a compartmentalized basin. The compartments (most often three) are separated by baffles to prevent short circuiting of the water being treated. The turbulence can be reduced gradually by reducing the speed of the mixers in each succeeding tank or by reducing the Surface area of the paddles. This is called tapered-energy mixing. The reason for reducing the speed of the stirrers is to prevent breaking apart the larger flocs, which have already formed. If the floc is broken up nothing is accomplished and the filter gets overloaded. | The flocculators may be circular, square or rectangular. The best flocculation is usually achieved in a compartmentalized basin. The compartments (most often three) are separated by baffles to prevent short circuiting of the water being treated. The turbulence can be reduced gradually by reducing the speed of the mixers in each succeeding tank or by reducing the Surface area of the paddles. This is called tapered-energy mixing. The reason for reducing the speed of the stirrers is to prevent breaking apart the larger flocs, which have already formed. If the floc is broken up nothing is accomplished and the filter gets overloaded. | ||
| − | (c) Coagulation – Flocculation Process Action | + | (c) '''Coagulation – Flocculation Process Action''' |
| + | |||
Typical jobs performed by an operator in the normal operation of the coagulation-flocculation process include the following: | Typical jobs performed by an operator in the normal operation of the coagulation-flocculation process include the following: | ||
| + | |||
(i) Monitor process performance, | (i) Monitor process performance, | ||
| + | |||
(ii) Evaluate water quality conditions (raw and treated water), | (ii) Evaluate water quality conditions (raw and treated water), | ||
| + | |||
(iii) Check and adjust process controls and equipment, and | (iii) Check and adjust process controls and equipment, and | ||
| + | |||
(iv) Visually inspect facilities. | (iv) Visually inspect facilities. | ||
| − | (d) Interaction with Sedimentation and Filtration | + | |
| + | (d) '''Interaction with Sedimentation and Filtration''' | ||
| + | |||
The processes of coagulation-flocculation are required to precondition or prepare non settle able particles present in the raw water for removal by sedimentation and filtration. Small particles (particularly colloids), without proper coagulation-flocculation are too light to settle out and will not be large enough to be trapped during filtration process. Since the purpose of coagulation–flocculation is to accelerate particle removal, the effectiveness of the sedimentation and filtration processes, as well as overall performance depends upon successful coagulation - flocculation. | The processes of coagulation-flocculation are required to precondition or prepare non settle able particles present in the raw water for removal by sedimentation and filtration. Small particles (particularly colloids), without proper coagulation-flocculation are too light to settle out and will not be large enough to be trapped during filtration process. Since the purpose of coagulation–flocculation is to accelerate particle removal, the effectiveness of the sedimentation and filtration processes, as well as overall performance depends upon successful coagulation - flocculation. | ||
| − | (e) Examination of the Floc | + | (e) '''Examination of the Floc''' |
| + | |||
• Examine the water samples at several points, en-route the flow line of the water. Look at the clarity of the water between the flocs and study the shape and size of the flocs. Observe the floc as it enters the flocculation basins which should be small and well dispersed throughout the flow; | • Examine the water samples at several points, en-route the flow line of the water. Look at the clarity of the water between the flocs and study the shape and size of the flocs. Observe the floc as it enters the flocculation basins which should be small and well dispersed throughout the flow; | ||
| + | |||
• Tiny alum floc may be an indication that the chemical dose is too low. A ‘popcorn flake’ is a desirable floc. If the water has a milky appearance or a bluish tint, the alum dose is probably too high. As the floc moves through the flocculation basins, the size of the floc should be increasing. If the size of the floc increases and then later starts to break up, the mixing intensity of the downstream flocculator may be too high. Thus, the speed of these flocculators needs to be reduced or otherwise the coagulant dosage may be increased; | • Tiny alum floc may be an indication that the chemical dose is too low. A ‘popcorn flake’ is a desirable floc. If the water has a milky appearance or a bluish tint, the alum dose is probably too high. As the floc moves through the flocculation basins, the size of the floc should be increasing. If the size of the floc increases and then later starts to break up, the mixing intensity of the downstream flocculator may be too high. Thus, the speed of these flocculators needs to be reduced or otherwise the coagulant dosage may be increased; | ||
| + | |||
• Examine the settlement of the floc in the sedimentation basin. If a lot of flocs are observed flowing over the laundering weirs the floc is too light for the detention time. By increasing the chemical dose or adding a coagulant aid such as a polymer to produce heavier and larger flocs. The appearance of the fine floc particles passing over the weir could be an indication of too much alum and the dose should be reduced. For precise evaluation only one change can be made at a time and evaluate the results. | • Examine the settlement of the floc in the sedimentation basin. If a lot of flocs are observed flowing over the laundering weirs the floc is too light for the detention time. By increasing the chemical dose or adding a coagulant aid such as a polymer to produce heavier and larger flocs. The appearance of the fine floc particles passing over the weir could be an indication of too much alum and the dose should be reduced. For precise evaluation only one change can be made at a time and evaluate the results. | ||
| − | (f) Record keeping | + | |
| + | (f) '''Record keeping''' | ||
| + | |||
Records of the following items should be maintained: | Records of the following items should be maintained: | ||
| + | |||
• Source water quality (pH, turbidity, temperature, alkalinity, chlorine demand and colour; | • Source water quality (pH, turbidity, temperature, alkalinity, chlorine demand and colour; | ||
| + | |||
• Process water quality (pH, turbidity, and alkalinity); | • Process water quality (pH, turbidity, and alkalinity); | ||
| + | |||
• Process production inventories (chemicals used, chemical feed rates, amount of water processed, and amount of chemicals in storage); | • Process production inventories (chemicals used, chemical feed rates, amount of water processed, and amount of chemicals in storage); | ||
| + | |||
• Process equipment performance (types of equipment in operation, maintenance procedures performed, equipment calibration and adjustments); | • Process equipment performance (types of equipment in operation, maintenance procedures performed, equipment calibration and adjustments); | ||
| + | |||
• A plot of key process variables should be maintained. A plot of source water turbidity vs. coagulant dosage should be maintained. If other process variables such as alkalinity or pH vary significantly, these should also be plotted. | • A plot of key process variables should be maintained. A plot of source water turbidity vs. coagulant dosage should be maintained. If other process variables such as alkalinity or pH vary significantly, these should also be plotted. | ||
| − | (g) Safety considerations | + | |
| + | (g) '''Safety considerations''' | ||
| + | |||
In the coagulation-flocculation processes, the operator may be exposed to the associated hazards with following: | In the coagulation-flocculation processes, the operator may be exposed to the associated hazards with following: | ||
| + | |||
• Electrical equipment, | • Electrical equipment, | ||
| + | |||
• Rotating mechanical equipment, | • Rotating mechanical equipment, | ||
| + | |||
• Water treatment chemicals, | • Water treatment chemicals, | ||
| + | |||
• Laboratory reagents (chemicals), | • Laboratory reagents (chemicals), | ||
| + | |||
• Slippery surfaces caused by certain chemicals, | • Slippery surfaces caused by certain chemicals, | ||
| + | |||
• Flooding, | • Flooding, | ||
| + | |||
• Confined spaces and underground structures such as valve or pump vaults (toxic and explosives gases, insufficient oxygen). | • Confined spaces and underground structures such as valve or pump vaults (toxic and explosives gases, insufficient oxygen). | ||
| + | |||
Strict and constant attention must be given to safety procedures. The operator must be trained with general first aid practices such as mouth-to-mouth resuscitation, treatment of common physical injuries, and first aid for chemical exposure (chlorine). | Strict and constant attention must be given to safety procedures. The operator must be trained with general first aid practices such as mouth-to-mouth resuscitation, treatment of common physical injuries, and first aid for chemical exposure (chlorine). | ||
| − | (h) Laboratory Tests | + | (h) '''Laboratory Tests''' |
| + | |||
Water quality indicators for the operation of flocculation process include turbidity, alkalinity, chlorine demand, residual chlorine test, colour, pH, temperature, odour and appearance and need to be tested. In multi-habitation or big schemes, a provision of automatic water testing equipment or onsite laboratory at treatment plant may be established and maintained for the purpose. | Water quality indicators for the operation of flocculation process include turbidity, alkalinity, chlorine demand, residual chlorine test, colour, pH, temperature, odour and appearance and need to be tested. In multi-habitation or big schemes, a provision of automatic water testing equipment or onsite laboratory at treatment plant may be established and maintained for the purpose. | ||
| − | + | ==== Rapid Sand Filtration Plant ==== | |
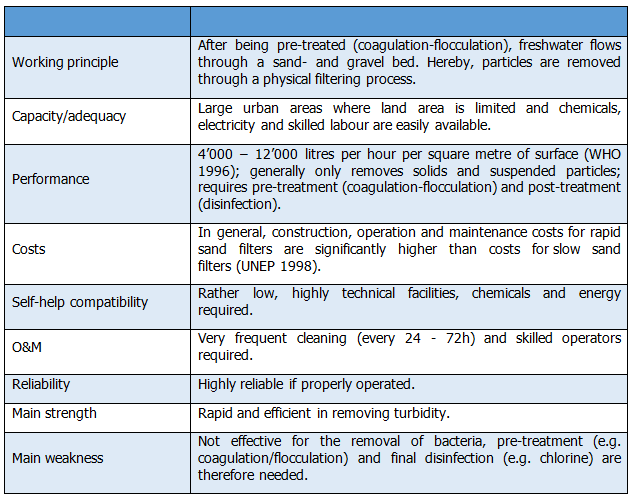
This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media which actually removes the particles from the water. The filter media is routinely cleaned by means of a backwashing process. | This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media which actually removes the particles from the water. The filter media is routinely cleaned by means of a backwashing process. | ||
| Line 418: | Line 457: | ||
Rapid sand filtration is a highly effective method to remove turbidity if it is correctly applied (Brikke & Bredero 2003). Equally, solids formed during pre-treatment, i.e. coagulation-flocculation, are filtered. A well-operated RSF reduces turbidity to less than 1 NTN and often less than 0.1 NTU (WHO 1996). Regarding the removal of most other contaminants, the RSFs are ineffective. If combined with adequate pre-treatment measures and final disinfection, rapid sand filtration usually produces safe drinking water. | Rapid sand filtration is a highly effective method to remove turbidity if it is correctly applied (Brikke & Bredero 2003). Equally, solids formed during pre-treatment, i.e. coagulation-flocculation, are filtered. A well-operated RSF reduces turbidity to less than 1 NTN and often less than 0.1 NTU (WHO 1996). Regarding the removal of most other contaminants, the RSFs are ineffective. If combined with adequate pre-treatment measures and final disinfection, rapid sand filtration usually produces safe drinking water. | ||
| − | (a) Filter sand | + | (a) '''Filter sand''' |
| + | |||
Filter sand is defined in terms of effective size and uniformity coefficient. Effective size is the sieve size in mm that permits 10% by weight to pass. Uniformity in size is specified by the uniformity coefficient which is the ratio between the sieve sizes that will pass 60% by weight and the effective size. | Filter sand is defined in terms of effective size and uniformity coefficient. Effective size is the sieve size in mm that permits 10% by weight to pass. Uniformity in size is specified by the uniformity coefficient which is the ratio between the sieve sizes that will pass 60% by weight and the effective size. | ||
| + | |||
Check shape size and quantity of filter sand to the followings: | Check shape size and quantity of filter sand to the followings: | ||
| + | |||
(i) Sand shall be of hard and resistant quartz or quartzite and free of clay, fine particles, soft grains and dirt of every description, | (i) Sand shall be of hard and resistant quartz or quartzite and free of clay, fine particles, soft grains and dirt of every description, | ||
| + | |||
(ii) Effective size shall be 0.4 to 0.7 mm, | (ii) Effective size shall be 0.4 to 0.7 mm, | ||
| + | |||
(iii) Uniformity coefficient shall not be more than 1.7 nor less than 1.3, | (iii) Uniformity coefficient shall not be more than 1.7 nor less than 1.3, | ||
| + | |||
(iv) Ignition loss should not exceed 0.7 per cent by weight, | (iv) Ignition loss should not exceed 0.7 per cent by weight, | ||
| + | |||
(v) Soluble fraction in hydrochloric acid shall not exceed 5.0% by weight, | (v) Soluble fraction in hydrochloric acid shall not exceed 5.0% by weight, | ||
| + | |||
(vi) Silica content should be not less than 90%, | (vi) Silica content should be not less than 90%, | ||
| + | |||
(vii) Specific gravity shall be in the range between 2.55 to 2.65, | (vii) Specific gravity shall be in the range between 2.55 to 2.65, | ||
| + | |||
(viii) Wearing loss shall not exceed 3%. | (viii) Wearing loss shall not exceed 3%. | ||
| − | + | ||
| + | ===== Interaction with Other Treatment Processes ===== | ||
The purpose of RSF is to remove particulate impurities and floc from the raw water. In this regard, the filtration process is the final step in the solids removal process which usually includes the pre-treatment processes of coagulation, flocculation and sedimentation. The degree of treatment applied prior to filtration depends on the quality of water. | The purpose of RSF is to remove particulate impurities and floc from the raw water. In this regard, the filtration process is the final step in the solids removal process which usually includes the pre-treatment processes of coagulation, flocculation and sedimentation. The degree of treatment applied prior to filtration depends on the quality of water. | ||
| − | Operation and Backwashing | + | ===== Operation and Backwashing ===== |
| + | |||
Rapid Sand Filters should be washed before placing them into service. | Rapid Sand Filters should be washed before placing them into service. | ||
(a) A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process should be stopped and the filter be taken out of service for cleaning or backwashing; | (a) A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process should be stopped and the filter be taken out of service for cleaning or backwashing; | ||
| + | |||
(b) The surface wash system should be activated just before the backwash cycle starts to aid in removing and breaking up solids on the filter media and to prevent the development of mud balls. The surface wash system should be stopped before completion of the back-wash cycle to permit proper settling of the filter media; | (b) The surface wash system should be activated just before the backwash cycle starts to aid in removing and breaking up solids on the filter media and to prevent the development of mud balls. The surface wash system should be stopped before completion of the back-wash cycle to permit proper settling of the filter media; | ||
| + | |||
(c) A filter wash should begin slowly for about one minute to permit removing of an entrapped air from the filter media, and also to provide uniform expansion of the filter bed. After this period, the full backwash rate can be applied. Sufficient time should be allowed for cleaning of the filter media. Usually when the backwash water coming up through the filter becomes clear, the media is washed. This generally takes from 3 to 8 minutes. If flooding of wash water troughs or carryover of filter media is a problem, the backwash rate must be reduced. | (c) A filter wash should begin slowly for about one minute to permit removing of an entrapped air from the filter media, and also to provide uniform expansion of the filter bed. After this period, the full backwash rate can be applied. Sufficient time should be allowed for cleaning of the filter media. Usually when the backwash water coming up through the filter becomes clear, the media is washed. This generally takes from 3 to 8 minutes. If flooding of wash water troughs or carryover of filter media is a problem, the backwash rate must be reduced. | ||
| + | |||
A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process is stopped and the filter is taken out of service for cleaning or backwashing. | A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process is stopped and the filter is taken out of service for cleaning or backwashing. | ||
| Line 446: | Line 500: | ||
Surface Wash: In order to produce optimum cleaning of the filter media during backwashing and to prevent mud balls, surface wash (supplemental scouring) is usually practiced. Surface wash systems provide additional scrubbing action to remove attached floc and other suspended solids from the filter media. | Surface Wash: In order to produce optimum cleaning of the filter media during backwashing and to prevent mud balls, surface wash (supplemental scouring) is usually practiced. Surface wash systems provide additional scrubbing action to remove attached floc and other suspended solids from the filter media. | ||
| − | + | ===== Operation and Maintenance of Rapid Sand Filters ===== | |
Operation of a rapid sand filter consists of flow control, regular backwashing and cleaning. The period between backwashes depends on the quality of the influent water and normally lies between 24 – 72 hours (UNEP 1998).The cleaning process requires an interruption of the purification process of 5 - 10 minutes per filter bed. Several parallel filter units are required to guarantee constant water supply. The backwash process must be observed carefully; in particular the rate of flow must be controlled to avoid erosion of the filter medium. Periodic repacking of the filter bed may be required at infrequent intervals to ensure efficient operation (UNEP 1998). Operation and maintenance thus requires skilled and highly reliable workers. Table 12.2 illustrate the details of RSF operation and maintenance. | Operation of a rapid sand filter consists of flow control, regular backwashing and cleaning. The period between backwashes depends on the quality of the influent water and normally lies between 24 – 72 hours (UNEP 1998).The cleaning process requires an interruption of the purification process of 5 - 10 minutes per filter bed. Several parallel filter units are required to guarantee constant water supply. The backwash process must be observed carefully; in particular the rate of flow must be controlled to avoid erosion of the filter medium. Periodic repacking of the filter bed may be required at infrequent intervals to ensure efficient operation (UNEP 1998). Operation and maintenance thus requires skilled and highly reliable workers. Table 12.2 illustrate the details of RSF operation and maintenance. | ||
| Line 453: | Line 507: | ||
Table 12. 2: Operation and Maintenance Details for RSF | Table 12. 2: Operation and Maintenance Details for RSF | ||
| − | + | [[File:12.22.png|700px|center]] | |
| − | |||
| − | |||
| − | |||
| − | + | ====== Operating Procedures ====== | |
| − | |||
| − | |||
| − | |||
| − | |||
| + | From a water quality point of view, filter effluent turbidity is a good indication of overall process performance. However, monitoring the performance of each of the individual water treatment process including sedimentation is must in order to check water quality or performance changes. Operations are considered to be normal within the operating ranges of the plant, while unusual or difficult to handle condition is abnormal operating condition. In normal operation of the sedimentation process one must monitor the following; | ||
| − | + | (a) ''Turbidity of inflow and out flow of Water in the Sedimentation Basin:'' Turbidity of inflow water indicates the floc or solids loading to the sedimentation basin while turbidity of outflow water of the basin indicates the effectiveness or efficiency of the sedimentation process. Low levels of outflow water turbidity to be achieved to minimize the floc loading on the filter. | |
| − | + | ||
| + | (b) ''Temperature of inflow water:'' is important as the water becomes colder, the settling of particles become slow. To compensate for this change, jar tests should be performed and accordingly, the coagulant dosage is to be adjusted to produce a heavier and thus a settle-able floc. Another possibility is to provide longer detention times when water demand decreases. | ||
| + | |||
| + | (c) ''Visual checks of the sedimentation process:'' should include observation of floc settling characteristics, distribution of floc at the basin inlet and clarity of outflow settled water spilling over the weirs. An uneven distribution of floc or poorly settling floc is an indication of a raw water quality change or there is operational problem. | ||
| − | + | (d) ''Process Actions/ steps are as indicated below:'' | |
| − | |||
| − | |||
| − | (d) Process Actions/ steps are as indicated below: | ||
(i) Monitor process performance. | (i) Monitor process performance. | ||
| + | |||
(ii) Evaluate turbidity and make appropriate process changes. | (ii) Evaluate turbidity and make appropriate process changes. | ||
| + | |||
(iii) Check and adjust processes equipment (change chemical feed rates). | (iii) Check and adjust processes equipment (change chemical feed rates). | ||
| + | |||
(iv) Backwash filters. | (iv) Backwash filters. | ||
| + | |||
(v) Evaluate filter media condition (media loss, mud balls, cracking), | (v) Evaluate filter media condition (media loss, mud balls, cracking), | ||
| + | |||
(vi) Visually inspect facilities. | (vi) Visually inspect facilities. | ||
| − | + | ||
| + | ====== Important Process Activities and Precautions ====== | ||
Process performance monitoring is an on-going activity. Check for any treatment process changes or other problems which might affect filtered water quality, such as a chemical feed system failure. Measurement of head-loss built up in the filter media may give a good indication of how well the solids removal process is performing. The total designed head loss from the filter influent to the effluent in a gravity filter is usually about 3 meters. At the beginning of the filtration cycle the actual measured head loss due to clean media and other hydraulic losses are about 0.9 m. This would permit an additional head-loss of about 2.1 m due to solid accumulation in the filter. | Process performance monitoring is an on-going activity. Check for any treatment process changes or other problems which might affect filtered water quality, such as a chemical feed system failure. Measurement of head-loss built up in the filter media may give a good indication of how well the solids removal process is performing. The total designed head loss from the filter influent to the effluent in a gravity filter is usually about 3 meters. At the beginning of the filtration cycle the actual measured head loss due to clean media and other hydraulic losses are about 0.9 m. This would permit an additional head-loss of about 2.1 m due to solid accumulation in the filter. | ||
| Line 488: | Line 542: | ||
In the filter process, time for completion of normal filter process may be calculated on the basis of the following parameters: | In the filter process, time for completion of normal filter process may be calculated on the basis of the following parameters: | ||
| + | |||
(a) Head-loss; | (a) Head-loss; | ||
| + | |||
(b) Effluent turbidity level; | (b) Effluent turbidity level; | ||
| + | |||
(c) Elapsed run time; | (c) Elapsed run time; | ||
| + | |||
(d) A predetermined value established for each above parameter as a cut off point for filter operation may be checked and when any of the selves is reached, the filter should be removed from service and backwashed; | (d) A predetermined value established for each above parameter as a cut off point for filter operation may be checked and when any of the selves is reached, the filter should be removed from service and backwashed; | ||
| + | |||
(e) At least once a year, the filter media must be examined and evaluate its overall condition; | (e) At least once a year, the filter media must be examined and evaluate its overall condition; | ||
| + | |||
(f) Measure the filter media thickness for an indication of media loss during the back-washing process; | (f) Measure the filter media thickness for an indication of media loss during the back-washing process; | ||
| + | |||
(g) Mud ball accumulation in the filter media to evaluate the effectiveness of the overall back-washing operation. | (g) Mud ball accumulation in the filter media to evaluate the effectiveness of the overall back-washing operation. | ||
| − | + | ||
| + | ====== Routine observations ====== | ||
| + | |||
(a) The backwash process to qualitatively assess process performance, | (a) The backwash process to qualitatively assess process performance, | ||
| + | |||
(b) For media boils (uneven flow distribution) during backwashing, media carry over in to the wash water trough, and | (b) For media boils (uneven flow distribution) during backwashing, media carry over in to the wash water trough, and | ||
| + | |||
(c) Clarity of the waste wash-water near the end of the backwash cycle, | (c) Clarity of the waste wash-water near the end of the backwash cycle, | ||
| + | |||
(d) Upon completion of the backwash cycle, observe the condition of the media surface, | (d) Upon completion of the backwash cycle, observe the condition of the media surface, | ||
| + | |||
(e) Check for filter sidewall or media surface cracks, | (e) Check for filter sidewall or media surface cracks, | ||
| + | |||
(f) Routinely inspect physical facilities, equipment as part of good house-keeping and maintenance practices, | (f) Routinely inspect physical facilities, equipment as part of good house-keeping and maintenance practices, | ||
| + | |||
(g) Correct or report the abnormal equipment conditions to the water supply utility/agency for maintenance action. | (g) Correct or report the abnormal equipment conditions to the water supply utility/agency for maintenance action. | ||
| + | |||
Never bump upon filter to avoid back-washing. Bumping is the act of opening the backwash valve during the course of a filter run to dislodge the trapped solids and increase the length of filter run. This is not a good practice. Shortened filter runs can occur because of air bound filters. Air binding will occur more frequently when large head losses are allowed to develop in the filter. Precautions should be taken to minimize air binding to avoid damage to the filter media. | Never bump upon filter to avoid back-washing. Bumping is the act of opening the backwash valve during the course of a filter run to dislodge the trapped solids and increase the length of filter run. This is not a good practice. Shortened filter runs can occur because of air bound filters. Air binding will occur more frequently when large head losses are allowed to develop in the filter. Precautions should be taken to minimize air binding to avoid damage to the filter media. | ||
| − | + | ====== Record Keeping ====== | |
| + | |||
A daily operations log of process performance data and water quality characteristics shall be recorded and maintained accurately for the following items: | A daily operations log of process performance data and water quality characteristics shall be recorded and maintained accurately for the following items: | ||
| + | |||
(a) Process water quality (turbidity, colour, PH and alkalinity); | (a) Process water quality (turbidity, colour, PH and alkalinity); | ||
| + | |||
(b) Process operation (filters in service, filtration rates, loss of head, length of filter runs, frequency of backwash, backwash rates, and UFRV unit filter run volume); | (b) Process operation (filters in service, filtration rates, loss of head, length of filter runs, frequency of backwash, backwash rates, and UFRV unit filter run volume); | ||
| + | |||
(c) Process water production (water processed, amount of backwash water used, and chemicals used); | (c) Process water production (water processed, amount of backwash water used, and chemicals used); | ||
| + | |||
(d) Percentage of water production used to backwash filters; | (d) Percentage of water production used to backwash filters; | ||
| + | |||
(e) Process equipment performance (types of equipment in operation, equipment adjustments, maintenance procedures performed, and equipment calibration). | (e) Process equipment performance (types of equipment in operation, equipment adjustments, maintenance procedures performed, and equipment calibration). | ||
| − | |||
| − | (a) Routine Procedures | + | ====== Start-up and Shutdown Procedures ====== |
| + | |||
| + | '''(a) Routine Procedures''' | ||
Most plants keep all filters into service except unit under backwash operation and maintenance. Filter units are routinely taken off line for backwashing when the media becomes clogged with particulates, turbidity break through occurs or demands for water are reduced. | Most plants keep all filters into service except unit under backwash operation and maintenance. Filter units are routinely taken off line for backwashing when the media becomes clogged with particulates, turbidity break through occurs or demands for water are reduced. | ||
| − | (b) Implementation of Start-up and Shut-down Procedures | + | '''(b) Implementation of Start-up and Shut-down Procedures''' |
| + | |||
Filter check-out procedures: | Filter check-out procedures: | ||
| + | |||
(i) Check operational status of filter; | (i) Check operational status of filter; | ||
| + | |||
(ii) Be sure that the filter media and wash water troughs are clean of all debris such as leaves, twigs, and tools; | (ii) Be sure that the filter media and wash water troughs are clean of all debris such as leaves, twigs, and tools; | ||
| − | (iii) Check and be sure that all access covers and walk-way gratings are in place; | + | |
| + | (iii) Check and be sure that all access covers and walk-way gratings are in place; | ||
| + | |||
(iv) Make sure that the process monitoring equipment such as head-loss and turbidity systems are operational; | (iv) Make sure that the process monitoring equipment such as head-loss and turbidity systems are operational; | ||
| + | |||
(v) Check the source of back-wash to ensure that it is ready to go. | (v) Check the source of back-wash to ensure that it is ready to go. | ||
| − | + | ||
| + | ====== Preventive Maintenance Procedures ====== | ||
Preventive maintenance programmes are to assure the continued satisfactory operation of treatment plant facilities by reducing the frequency of break-down failures. Routine maintenance functions of operator may include: | Preventive maintenance programmes are to assure the continued satisfactory operation of treatment plant facilities by reducing the frequency of break-down failures. Routine maintenance functions of operator may include: | ||
| + | |||
(i) Keeping electric motors free of dirt, moisture and pests (rodent sand birds); | (i) Keeping electric motors free of dirt, moisture and pests (rodent sand birds); | ||
| + | |||
(ii) Assuming good ventilation (air circulation) in equipment work areas; | (ii) Assuming good ventilation (air circulation) in equipment work areas; | ||
| + | |||
(iii) Checking pumps and motors for leaks, unusual noise and vibrations or overheating; | (iii) Checking pumps and motors for leaks, unusual noise and vibrations or overheating; | ||
| + | |||
(iv) Maintaining proper lubrication and oil levels; | (iv) Maintaining proper lubrication and oil levels; | ||
| + | |||
(v) Inspecting for alignment of shafts and couplings; | (v) Inspecting for alignment of shafts and couplings; | ||
| + | |||
(vi) Checking bearings for overheating and proper lubrication; | (vi) Checking bearings for overheating and proper lubrication; | ||
| + | |||
(vii) Checking the proper valve operation (leakage or jamming); | (vii) Checking the proper valve operation (leakage or jamming); | ||
| + | |||
(viii) Checking automatic control systems for proper operation; | (viii) Checking automatic control systems for proper operation; | ||
| + | |||
(ix) Checking air/vacuum relief systems for proper functioning, dirt and moisture; | (ix) Checking air/vacuum relief systems for proper functioning, dirt and moisture; | ||
| + | |||
(x) Verifying correct operation of filters and back-washing cycles by observation; | (x) Verifying correct operation of filters and back-washing cycles by observation; | ||
| + | |||
(xi) Inspecting filter media conditions (look for algae and mud balls and examine gravel and media for proper gradation); | (xi) Inspecting filter media conditions (look for algae and mud balls and examine gravel and media for proper gradation); | ||
| + | |||
(xii) Inspecting filter underdrain system (be sure that the under drain openings are not becoming clogged due to media, corrosion nor chemical deposits). | (xii) Inspecting filter underdrain system (be sure that the under drain openings are not becoming clogged due to media, corrosion nor chemical deposits). | ||
| − | |||
| − | (a) Electrical Equipment | + | ====== Safety Considerations ====== |
| + | |||
| + | '''(a) Electrical Equipment''' | ||
| + | |||
(i) Avoid electric shock (use preventive gloves), | (i) Avoid electric shock (use preventive gloves), | ||
| + | |||
(ii) Avoid grounding yourself in water or on pipes, | (ii) Avoid grounding yourself in water or on pipes, | ||
| + | |||
(iii) Ground all electric tools, | (iii) Ground all electric tools, | ||
| + | |||
(iv) Lock-out and tag electrical switches and panels when servicing equipment. | (iv) Lock-out and tag electrical switches and panels when servicing equipment. | ||
| − | (b) Mechanical Equipment | + | |
| + | '''(b) Mechanical Equipment''' | ||
| + | |||
(i) Use protective guards on rotating equipment, | (i) Use protective guards on rotating equipment, | ||
| + | |||
(ii) Don’t wear loose clothing around rotating equipment, | (ii) Don’t wear loose clothing around rotating equipment, | ||
| + | |||
(iii) Keep hands out of energized valves, pumps and other pieces of equipment, | (iii) Keep hands out of energized valves, pumps and other pieces of equipment, | ||
| + | |||
(iv) Clean –up all lubricant and chemicals pills (slippery surfaces cause bad falls). | (iv) Clean –up all lubricant and chemicals pills (slippery surfaces cause bad falls). | ||
| − | (c) Open – Surface Filter | + | |
| + | '''(c) Open – Surface Filter''' | ||
| + | |||
(i) Use safety devices such as hand rails and ladders, | (i) Use safety devices such as hand rails and ladders, | ||
| + | |||
(ii) Close all openings and replace safety gratings when finished working, | (ii) Close all openings and replace safety gratings when finished working, | ||
| + | |||
(iii) Know the location of all life preservers and other safety devices. | (iii) Know the location of all life preservers and other safety devices. | ||
| − | (d) Valve and Pump Vaults, Sumps, Filter galleries | + | |
| + | '''(d) Valve and Pump Vaults, Sumps, Filter galleries''' | ||
| + | |||
(i) Be sure that all underground or confined structures are free of hazardous atmospheres (toxic or explosive gases, lack of oxygen)by checking with gas detectors, | (i) Be sure that all underground or confined structures are free of hazardous atmospheres (toxic or explosive gases, lack of oxygen)by checking with gas detectors, | ||
| + | |||
(ii) Work in well ventilated structures (use air circulation fans). | (ii) Work in well ventilated structures (use air circulation fans). | ||
| − | + | ==== Sedimentation ==== | |
| − | |||
Sedimentation tank, also called settling tank or clarifier, component of a modern system of water supply or wastewater treatment. A sedimentation tank allows suspended particles to settle out of water or wastewater as it flows slowly through the tank, thereby providing some degree of purification. The purpose of sedimentation process is to remove suspended particles so as to reduce load on Filters. If adequate detention time and basin surface area are provided in the sedimentation basins, solids removal efficiencies can be achieved more than 95%. However, it may not always be the cost effective way to remove suspended solids. | Sedimentation tank, also called settling tank or clarifier, component of a modern system of water supply or wastewater treatment. A sedimentation tank allows suspended particles to settle out of water or wastewater as it flows slowly through the tank, thereby providing some degree of purification. The purpose of sedimentation process is to remove suspended particles so as to reduce load on Filters. If adequate detention time and basin surface area are provided in the sedimentation basins, solids removal efficiencies can be achieved more than 95%. However, it may not always be the cost effective way to remove suspended solids. | ||
| Line 574: | Line 686: | ||
In low turbid water sources (less than about 10 NTU) effective coagulation, flocculation and filtration may produce satisfactory filtered water without sedimentation. In this case, coagulation-flocculation process is operated to produce a highly filterable tiny floc, which does not readily settle due to its small size; instead the tiny floc is removed by the filters. There is, however, a practical limitation in applying this concept to higher turbidity conditions. If the filters become overloaded with suspended solids, they will quickly clog and need frequent back washing. This can limit plant production and cause degradation in filtered water quality. Thus, the sedimentation process should be operated from the standpoint of overall plant efficiency. If the source water turbidity is only 3 mg/l, and the jar tests indicate that 0.5 mg/l of coagulant is the most effective dosage, then one cannot expect the sedimentation process to remove a significant fraction of the suspended solids. On the other hand, source water turbidity in excess of 50 mg/l will probably require a high coagulant dosage for efficient solids removal and the suspended particles and alum floc should be removed by sedimentation basin. | In low turbid water sources (less than about 10 NTU) effective coagulation, flocculation and filtration may produce satisfactory filtered water without sedimentation. In this case, coagulation-flocculation process is operated to produce a highly filterable tiny floc, which does not readily settle due to its small size; instead the tiny floc is removed by the filters. There is, however, a practical limitation in applying this concept to higher turbidity conditions. If the filters become overloaded with suspended solids, they will quickly clog and need frequent back washing. This can limit plant production and cause degradation in filtered water quality. Thus, the sedimentation process should be operated from the standpoint of overall plant efficiency. If the source water turbidity is only 3 mg/l, and the jar tests indicate that 0.5 mg/l of coagulant is the most effective dosage, then one cannot expect the sedimentation process to remove a significant fraction of the suspended solids. On the other hand, source water turbidity in excess of 50 mg/l will probably require a high coagulant dosage for efficient solids removal and the suspended particles and alum floc should be removed by sedimentation basin. | ||
| − | + | ||
| + | ===== Sedimentation Basins ===== | ||
The Basin can be divided into four zones viz. Inlet; Settling; Sludge and Outlet zone. The basins may be of the following types: | The Basin can be divided into four zones viz. Inlet; Settling; Sludge and Outlet zone. The basins may be of the following types: | ||
| + | |||
(a) Rectangular basins, | (a) Rectangular basins, | ||
| + | |||
(b) Circular and square basins, | (b) Circular and square basins, | ||
| + | |||
(c) High Rate Settlers (Tube Settlers), | (c) High Rate Settlers (Tube Settlers), | ||
| + | |||
(d) Solid Contact Units (Up-flow solid-contact clarification and up-flow sludge blanket clarification). | (d) Solid Contact Units (Up-flow solid-contact clarification and up-flow sludge blanket clarification). | ||
| − | + | ||
| + | ===== Process Actions ===== | ||
In rectangular and circular sedimentation basins, it is generally possible to make a judgment about the performance of the sedimentation process by observing how far the flocs are visible beyond the basin inlet. When sedimentation is working well, the floc will only be visible for short distance. When the sedimentation is poor, the floc will be visible for a long distance beyond the inlet. | In rectangular and circular sedimentation basins, it is generally possible to make a judgment about the performance of the sedimentation process by observing how far the flocs are visible beyond the basin inlet. When sedimentation is working well, the floc will only be visible for short distance. When the sedimentation is poor, the floc will be visible for a long distance beyond the inlet. | ||
| Line 590: | Line 708: | ||
With any of the sedimentation processes, it is useful to observe the quality of the effluent as it passes over the weir. Flocs coming over at the ends of the basin are indicative of density currents, short circuiting, sludge blankets that are too deep or high flows. The clarity of the outflow is also a reliable indicator of coagulation-flocculation efficiency. Process equipment should be checked regularly to assure adequate performance. Proper operation of sludge removal equipment should be verified each time for its operation, since sludge removal piping systems are subject to clogging. Free flowing sludge can be readily observed if sight glasses are incorporated in the sludge discharge piping. Otherwise, the outlet of the sludge line should be observed during sludge pumping. Frequent clogging of sludge pipe requires increasing frequency of sludge removal equipment and this can be diagnosed by performing sludge solids volume analysis in the laboratory. | With any of the sedimentation processes, it is useful to observe the quality of the effluent as it passes over the weir. Flocs coming over at the ends of the basin are indicative of density currents, short circuiting, sludge blankets that are too deep or high flows. The clarity of the outflow is also a reliable indicator of coagulation-flocculation efficiency. Process equipment should be checked regularly to assure adequate performance. Proper operation of sludge removal equipment should be verified each time for its operation, since sludge removal piping systems are subject to clogging. Free flowing sludge can be readily observed if sight glasses are incorporated in the sludge discharge piping. Otherwise, the outlet of the sludge line should be observed during sludge pumping. Frequent clogging of sludge pipe requires increasing frequency of sludge removal equipment and this can be diagnosed by performing sludge solids volume analysis in the laboratory. | ||
| − | + | ===== Sludge Management ===== | |
| + | |||
| + | ====== Sludge characteristics ====== | ||
| − | |||
Water treatment sludge is typically alum sludge, with solid concentrations varying from 0.25 to 10% when removed from a basin. In gravity flow sludge removal systems, the solid concentration should be limited to about 3%. If the sludge is to be pumped, solids concentrations should be high as 10% for readily transportation. In horizontal flow sedimentation basins preceded by coagulation and flocculation, over 50% of the floc will settle out in the first third of the basin length. Operationally, this must be considered when establishing the frequency of the operation of sludge removal equipment. | Water treatment sludge is typically alum sludge, with solid concentrations varying from 0.25 to 10% when removed from a basin. In gravity flow sludge removal systems, the solid concentration should be limited to about 3%. If the sludge is to be pumped, solids concentrations should be high as 10% for readily transportation. In horizontal flow sedimentation basins preceded by coagulation and flocculation, over 50% of the floc will settle out in the first third of the basin length. Operationally, this must be considered when establishing the frequency of the operation of sludge removal equipment. | ||
| − | + | ====== Sludge Removal Systems ====== | |
| + | |||
Sludge which accumulates on the bottom of the sedimentation basins must be removed periodically for the following reasons: | Sludge which accumulates on the bottom of the sedimentation basins must be removed periodically for the following reasons: | ||
| + | |||
• To prevent interference with the settling process (such as re-suspension of solids due to scouring); | • To prevent interference with the settling process (such as re-suspension of solids due to scouring); | ||
| + | |||
• To prevent the sludge from becoming septic or providing an environment for the growth of microorganisms that create taste and odour problems; | • To prevent the sludge from becoming septic or providing an environment for the growth of microorganisms that create taste and odour problems; | ||
| + | |||
• To prevent excessive reduction in the cross sectional area of the basin (reduction of detention time). | • To prevent excessive reduction in the cross sectional area of the basin (reduction of detention time). | ||
| + | |||
In large scale plants, sludge is normally removed on an intermittent basis with the aid of mechanical sludge removal equipment. However, in smaller plants with low solid loading, manual sludge removal may be more cost effective. In manually cleaned basins, the sludge is allowed to accumulate until it reduces settled water quality. High levels of sludge reduce the detention time and floc carries over to the filters. The basin is then dewatered (drained), most of the sludge is removed by stationary or portable pumps, and the remaining sludge is removed with squeegees and hoses. Basin floors are usually sloped towards a drain to help sludge removal. The frequency of shutdown for cleaning will vary from several months to a year or more, depending on source water quality (amount of suspended matter in the water). | In large scale plants, sludge is normally removed on an intermittent basis with the aid of mechanical sludge removal equipment. However, in smaller plants with low solid loading, manual sludge removal may be more cost effective. In manually cleaned basins, the sludge is allowed to accumulate until it reduces settled water quality. High levels of sludge reduce the detention time and floc carries over to the filters. The basin is then dewatered (drained), most of the sludge is removed by stationary or portable pumps, and the remaining sludge is removed with squeegees and hoses. Basin floors are usually sloped towards a drain to help sludge removal. The frequency of shutdown for cleaning will vary from several months to a year or more, depending on source water quality (amount of suspended matter in the water). | ||
In larger plants, a variety of mechanical devices can be used to remove sludge including: | In larger plants, a variety of mechanical devices can be used to remove sludge including: | ||
| + | |||
• Mechanical rakes, | • Mechanical rakes, | ||
| + | |||
• Drag-chain and flights, | • Drag-chain and flights, | ||
| + | |||
• Travelling bridge. | • Travelling bridge. | ||
| + | |||
Circular or square basins are usually equipped with rotating sludge rakes. Basin floors are sloped towards the centre and the sludge rakes progressively push the sludge toward a centre outlet. In rectangular basins, the simplest sludge removal mechanism is the chain and flight system. | Circular or square basins are usually equipped with rotating sludge rakes. Basin floors are sloped towards the centre and the sludge rakes progressively push the sludge toward a centre outlet. In rectangular basins, the simplest sludge removal mechanism is the chain and flight system. | ||
| − | + | ====== Sludge Disposal ====== | |
| + | |||
Disposal of waste from the water treatment plants has become increasingly important with the availability of technology and the need for protection of the environment. Treatment of waste solid adds to the cost of construction and operation of treatment plants. | Disposal of waste from the water treatment plants has become increasingly important with the availability of technology and the need for protection of the environment. Treatment of waste solid adds to the cost of construction and operation of treatment plants. | ||
| + | |||
Waste from the Water treatment plants comprise of: | Waste from the Water treatment plants comprise of: | ||
| + | |||
• Sludge from sedimentation of particulate matter in raw water, flocculated and precipitated material resulting from chemical coagulation, or residuals of excess chemical dosages, plankton, etc., | • Sludge from sedimentation of particulate matter in raw water, flocculated and precipitated material resulting from chemical coagulation, or residuals of excess chemical dosages, plankton, etc., | ||
| + | |||
• Waste from rinsing backwashing of filter media containing debris, chemical precipitates, straining of organic debris and plankton and residual of excess chemical dosages, etc.; and | • Waste from rinsing backwashing of filter media containing debris, chemical precipitates, straining of organic debris and plankton and residual of excess chemical dosages, etc.; and | ||
| + | |||
• Waster from regeneration processes of ion exchange softening treatment plant containing cation of calcium, magnesium and unused sodium and anion of chlorides and sulphates originally present in the regenerate. | • Waster from regeneration processes of ion exchange softening treatment plant containing cation of calcium, magnesium and unused sodium and anion of chlorides and sulphates originally present in the regenerate. | ||
| − | + | ||
| + | ====== Disposal Method ====== | ||
| + | |||
In continuous sludge removal, the feasibility of discharging of water treatment plant sludge to existing sewer nearby should be considered. For lime softening plant sludge, the reclamation by calcining and reuse can be explored. These sludge from clarification units using irons and aluminium coagulant can be dewatered by vacuum filtration. However the method of waste disposal shall conform to the pollution control norms. | In continuous sludge removal, the feasibility of discharging of water treatment plant sludge to existing sewer nearby should be considered. For lime softening plant sludge, the reclamation by calcining and reuse can be explored. These sludge from clarification units using irons and aluminium coagulant can be dewatered by vacuum filtration. However the method of waste disposal shall conform to the pollution control norms. | ||
| − | + | ====== Reuse of Sludge ====== | |
| + | |||
A large quantity of sludge is generated each year from water treatment plants in Tanzania. Disposing the sludge to the nearest watercourse is the common practice, especially by many urban water utilities, which accumulatively rise the aluminum concentrations in water and consequently in human bodies. Landfill disposal of the sludge is impractical because of the high cost of transportation and depletes the capacity of the landfill. The use of sludge in construction industry is considered to be the most economic and environmentally sound option. Due to the similar mineralogical composition of clay and water treatment plant sludge, various researchers have studied on the reuse of sludge in clay-brick production as a partial substitute for clay in brick manufacturing. However, concluded that by operating at the temperature commonly practiced in the brick kiln, 50 percent was the optimum sludge addition to produce brick from sludge-clay mixture. The produced bricks properties have proved superior to those available in the market. | A large quantity of sludge is generated each year from water treatment plants in Tanzania. Disposing the sludge to the nearest watercourse is the common practice, especially by many urban water utilities, which accumulatively rise the aluminum concentrations in water and consequently in human bodies. Landfill disposal of the sludge is impractical because of the high cost of transportation and depletes the capacity of the landfill. The use of sludge in construction industry is considered to be the most economic and environmentally sound option. Due to the similar mineralogical composition of clay and water treatment plant sludge, various researchers have studied on the reuse of sludge in clay-brick production as a partial substitute for clay in brick manufacturing. However, concluded that by operating at the temperature commonly practiced in the brick kiln, 50 percent was the optimum sludge addition to produce brick from sludge-clay mixture. The produced bricks properties have proved superior to those available in the market. | ||
(Source: https://www.researchgate.net/publication/295548404_Reuse_of_Water_Treatment_Plant_Sludge_in_Brick_Manufacturing) | (Source: https://www.researchgate.net/publication/295548404_Reuse_of_Water_Treatment_Plant_Sludge_in_Brick_Manufacturing) | ||
| − | + | ||
| + | ===== Start-up and Shutdown Procedures ===== | ||
In the event of requirement for shut down or start-up of processes on account of maintenance or a major equipment failure, proper procedures must be followed as per recommendations of the manufacturer of the plant and equipment. The procedures, in general, are given below: | In the event of requirement for shut down or start-up of processes on account of maintenance or a major equipment failure, proper procedures must be followed as per recommendations of the manufacturer of the plant and equipment. The procedures, in general, are given below: | ||
(a) Start up Procedure | (a) Start up Procedure | ||
| + | |||
(i) Check operational status, mode of operation of equipment and physical facilities: | (i) Check operational status, mode of operation of equipment and physical facilities: | ||
| + | |||
• Check that basin valves are closed, | • Check that basin valves are closed, | ||
| + | |||
• Check that basin isolation gates are closed, | • Check that basin isolation gates are closed, | ||
| + | |||
• Check that launder weir plates are set at equal elevations, | • Check that launder weir plates are set at equal elevations, | ||
| + | |||
• Check to ensure that all trash, debris and tools have been removed from basin. | • Check to ensure that all trash, debris and tools have been removed from basin. | ||
| + | |||
(ii) Test sludge removal equipment: | (ii) Test sludge removal equipment: | ||
| + | |||
• Check that mechanical equipment is properly lubricated and ready for operation, | • Check that mechanical equipment is properly lubricated and ready for operation, | ||
| + | |||
• Observe operation of sludge removal equipment. | • Observe operation of sludge removal equipment. | ||
| + | |||
(iii) Sedimentation basin filled with water: | (iii) Sedimentation basin filled with water: | ||
| + | |||
• Observe proper depth of water in basin, | • Observe proper depth of water in basin, | ||
| + | |||
• Remove floating debris from basin water surface. | • Remove floating debris from basin water surface. | ||
| + | |||
(iv) Start sample pumps, | (iv) Start sample pumps, | ||
| + | |||
(v) 5) Perform water quality analyses, | (v) 5) Perform water quality analyses, | ||
| + | |||
(vi) Operate sludge removal equipment. Be sure that all valves are in the proper position & operational. | (vi) Operate sludge removal equipment. Be sure that all valves are in the proper position & operational. | ||
| + | |||
(b) Shut down Procedures | (b) Shut down Procedures | ||
| + | |||
(i) Stop flow to sedimentation basin. Install basin isolation gates, | (i) Stop flow to sedimentation basin. Install basin isolation gates, | ||
| + | |||
(ii) Turn off sample pump, | (ii) Turn off sample pump, | ||
| + | |||
(iii) Turn off sludge removal equipment, | (iii) Turn off sludge removal equipment, | ||
| + | |||
(iv) Shut off mechanical equipment and disconnect where appropriate, | (iv) Shut off mechanical equipment and disconnect where appropriate, | ||
| + | |||
(v) Check that valves are in proper position& operational, | (v) Check that valves are in proper position& operational, | ||
| + | |||
(vi) Lock out electrical switches and equipment, | (vi) Lock out electrical switches and equipment, | ||
| + | |||
(vii) Dewater basin, if necessary; | (vii) Dewater basin, if necessary; | ||
| + | |||
(viii) Be sure that the water table is not high enough to float the empty basin. | (viii) Be sure that the water table is not high enough to float the empty basin. | ||
| + | |||
(ix) Open basin drain valves, | (ix) Open basin drain valves, | ||
| + | |||
(x) Grease and lubricate all gears, sprockets and mechanical moving parts which have been submerged immediately following dewatering to avoid seize up. | (x) Grease and lubricate all gears, sprockets and mechanical moving parts which have been submerged immediately following dewatering to avoid seize up. | ||
| − | |||
| − | (a) Types of support equipment – Operation and Maintenance | + | ===== Equipment ===== |
| + | |||
| + | '''(a) Types of support equipment – Operation and Maintenance''' | ||
| + | |||
The operator should be thoroughly familiar with the operation and maintenance instructions issued by the manufacturer for each specific equipment viz. flow meters and gauges valves control systems; water quality monitors such as turbidity meters; sludge removal equipment; sludge and sump pumps. | The operator should be thoroughly familiar with the operation and maintenance instructions issued by the manufacturer for each specific equipment viz. flow meters and gauges valves control systems; water quality monitors such as turbidity meters; sludge removal equipment; sludge and sump pumps. | ||
| − | Equipment Operation | + | '''Equipment Operation''' |
| + | |||
(i) Check the following: Proper lubrication and operational status of each unit, | (i) Check the following: Proper lubrication and operational status of each unit, | ||
| + | |||
(ii) Excessive noise and vibration, overheating and leakage, | (ii) Excessive noise and vibration, overheating and leakage, | ||
| + | |||
(iii) Pumps suction and discharge pressure. | (iii) Pumps suction and discharge pressure. | ||
| − | |||
| − | (a) Electrical Equipment | + | ===== Safety Considerations ===== |
| + | |||
| + | (a) '''Electrical Equipment''' | ||
| + | |||
(i) Avoid electric shock, | (i) Avoid electric shock, | ||
| + | |||
(ii) Avoid grounding yourself in water or on pipes, | (ii) Avoid grounding yourself in water or on pipes, | ||
| + | |||
(iii) Ground all electric tools, | (iii) Ground all electric tools, | ||
| + | |||
(iv) Use a lock out and tag system for electric equipment or electrically driven mechanical equipment. | (iv) Use a lock out and tag system for electric equipment or electrically driven mechanical equipment. | ||
| − | (b) (ii) Mechanical Equipment | + | |
| + | (b) '''(ii) Mechanical Equipment''' | ||
| + | |||
(i) Keep protective guards on rotating equipment, | (i) Keep protective guards on rotating equipment, | ||
| + | |||
(ii) Do not wear loose clothing around rotating equipment, | (ii) Do not wear loose clothing around rotating equipment, | ||
| + | |||
(iii) Keep hands out of valves, pumps and other equipment, | (iii) Keep hands out of valves, pumps and other equipment, | ||
| + | |||
(iv) Clean up all lubricant and sludge spills. | (iv) Clean up all lubricant and sludge spills. | ||
| − | (c) (iii) Open Surface water – filled structures | + | |
| + | (c) '''(iii) Open Surface water – filled structures''' | ||
| + | |||
(i) Use safety devices such as hand rails and ladders, | (i) Use safety devices such as hand rails and ladders, | ||
| + | |||
(ii) Close all openings, | (ii) Close all openings, | ||
| + | |||
(iii) Know the location of all life preservers. | (iii) Know the location of all life preservers. | ||
| − | (d) Valve and Pump Vaults, Sumps | + | |
| + | (d) '''Valve and Pump Vaults, Sumps''' | ||
| + | |||
(i) Be sure all underground or confined structures are free of hazardous atmosphere (Toxic or explosive gases, lack of oxygen), | (i) Be sure all underground or confined structures are free of hazardous atmosphere (Toxic or explosive gases, lack of oxygen), | ||
| + | |||
(ii) Work only in well ventilated structures, | (ii) Work only in well ventilated structures, | ||
| + | |||
(iii) Take proper steps against flooding. | (iii) Take proper steps against flooding. | ||
| − | + | ||
| + | ===== Corrosion Control ===== | ||
All metallic parts which are prone to corrosion must be protected. Corrosion can be controlled to a large extent by applying anti corrosive paints on the steel pipes at the time of construction of the borehole. Non-corrosive casing pipe and strainers (Such as PVC pipes and strainers) can also be used at the time of construction of borehole to avoid corrosion. Some commonly used paints/coatings to control corrosion are of aluminium, asphalt, red lead and coal tar. | All metallic parts which are prone to corrosion must be protected. Corrosion can be controlled to a large extent by applying anti corrosive paints on the steel pipes at the time of construction of the borehole. Non-corrosive casing pipe and strainers (Such as PVC pipes and strainers) can also be used at the time of construction of borehole to avoid corrosion. Some commonly used paints/coatings to control corrosion are of aluminium, asphalt, red lead and coal tar. | ||
| − | + | ||
| + | |||
| + | ===== Preventive Maintenance ===== | ||
Such programmes are designed to assure the continued satisfactory operation of treatment plant by reducing the frequency of breakdown failures. Typical steps should include: | Such programmes are designed to assure the continued satisfactory operation of treatment plant by reducing the frequency of breakdown failures. Typical steps should include: | ||
| + | |||
(a) Keeping electric motors free of dirt and moisture; | (a) Keeping electric motors free of dirt and moisture; | ||
| + | |||
(b) Assuring good ventilation at valve and pump vaults, sumps; | (b) Assuring good ventilation at valve and pump vaults, sumps; | ||
| + | |||
(c) Checking pumps and motors for leaks, unusual noise and vibrations, overheating or signs of wear; | (c) Checking pumps and motors for leaks, unusual noise and vibrations, overheating or signs of wear; | ||
| + | |||
(d) Maintaining proper lubrication and oil levels; | (d) Maintaining proper lubrication and oil levels; | ||
| + | |||
(e) Inspecting alignment of shafts and couplings; | (e) Inspecting alignment of shafts and couplings; | ||
| + | |||
(f) Checking bearings for overheating and proper lubrication; | (f) Checking bearings for overheating and proper lubrication; | ||
| + | |||
(g) Checking for proper valve operation; | (g) Checking for proper valve operation; | ||
| + | |||
(h) Checking for free flow of sludge in sludge removal collection and discharge systems; | (h) Checking for free flow of sludge in sludge removal collection and discharge systems; | ||
| + | |||
(i) Good housekeeping. | (i) Good housekeeping. | ||
| − | + | ||
| + | ==== Operation and Maintenance for Defluoridation ==== | ||
Fluoride compounds, usually calcium fluoride, are naturally found, usually in low concentration in water. However, water from underground sources can have higher levels of fluoride to the level that it becomes a health hazard. Defluoridation process is both difficult and expensive, more details and standards can be found in Volume I. | Fluoride compounds, usually calcium fluoride, are naturally found, usually in low concentration in water. However, water from underground sources can have higher levels of fluoride to the level that it becomes a health hazard. Defluoridation process is both difficult and expensive, more details and standards can be found in Volume I. | ||
| Line 717: | Line 915: | ||
For the absorption method, the following are the procedures: | For the absorption method, the following are the procedures: | ||
| + | |||
(a) Check filter against growth of algae (if exposed to sunlight), | (a) Check filter against growth of algae (if exposed to sunlight), | ||
| + | |||
(b) Check blocked filter by sedimentation, | (b) Check blocked filter by sedimentation, | ||
| + | |||
(c) Check fluoride saturation, | (c) Check fluoride saturation, | ||
| + | |||
(d) Check treated water quality (fluoride concentration) once per quarter. | (d) Check treated water quality (fluoride concentration) once per quarter. | ||
| − | |||
| − | + | === Tertiary Treatment === | |
| + | |||
| + | ==== Disinfection ==== | ||
Drinking water is disinfected to kill bacteria, viruses and parasites, which may exist in the water and may cause illness and disease like Campylobacter, Cholera, Amoebic Dysentery, Giardia (beaver fever) and Cryptosporidium. These organisms usually get into drinking water supplies when source of waters such as lakes or streams, community water transmission pipes or storage reservoirs are contaminated by animal waste or human sewage. Generally, deep wells are safer than shallow wells if chemical contamination is absent. In fact, shallow dug wells are often as contaminated as lakes or streams. The disinfection of potable water is almost universally accomplished by the use of gaseous chlorine or chlorine compounds. Chlorine is easy to apply, measure and control. It persists reasonably well and it is relatively inexpensive. Other methods of disinfection are also available viz. ozone, ultra-violet light, chlorine dioxide, silver ionization. | Drinking water is disinfected to kill bacteria, viruses and parasites, which may exist in the water and may cause illness and disease like Campylobacter, Cholera, Amoebic Dysentery, Giardia (beaver fever) and Cryptosporidium. These organisms usually get into drinking water supplies when source of waters such as lakes or streams, community water transmission pipes or storage reservoirs are contaminated by animal waste or human sewage. Generally, deep wells are safer than shallow wells if chemical contamination is absent. In fact, shallow dug wells are often as contaminated as lakes or streams. The disinfection of potable water is almost universally accomplished by the use of gaseous chlorine or chlorine compounds. Chlorine is easy to apply, measure and control. It persists reasonably well and it is relatively inexpensive. Other methods of disinfection are also available viz. ozone, ultra-violet light, chlorine dioxide, silver ionization. | ||
| − | + | ==== Chlorination ==== | |
The primary objectives of the chlorination process are disinfection, taste and odour control in the system, preventing the growth of algae and other micro-organisms that might interfere with coagulation and flocculation, keeping filter media free of slime growths and mud balls and preventing possible built up of anaerobic bacteria in the filter media, destroying hydrogen sulphide and controlling sulphurous taste and odour in the finished water, removing iron and manganese, bleaching of organic colour. | The primary objectives of the chlorination process are disinfection, taste and odour control in the system, preventing the growth of algae and other micro-organisms that might interfere with coagulation and flocculation, keeping filter media free of slime growths and mud balls and preventing possible built up of anaerobic bacteria in the filter media, destroying hydrogen sulphide and controlling sulphurous taste and odour in the finished water, removing iron and manganese, bleaching of organic colour. | ||
| Line 736: | Line 939: | ||
Dosage: Effective chlorine dose should be such that sufficient chlorine is there to react with organic matter, ammonia, iron, manganese and other reducing substances in water and at the same time leave sufficient chlorine to act as algaecide. Dose required for this purpose may be over 2mg/L Post chlorination dose can be adjusted to obtain minimum 0.2to 0.5 mg/l residual chlorine in potable water at consumer end. | Dosage: Effective chlorine dose should be such that sufficient chlorine is there to react with organic matter, ammonia, iron, manganese and other reducing substances in water and at the same time leave sufficient chlorine to act as algaecide. Dose required for this purpose may be over 2mg/L Post chlorination dose can be adjusted to obtain minimum 0.2to 0.5 mg/l residual chlorine in potable water at consumer end. | ||
| − | + | ===== Effectiveness of Chlorination ===== | |
Generally, chlorination without filtration or other pre-treatment is effective and adequate only under the following conditions: | Generally, chlorination without filtration or other pre-treatment is effective and adequate only under the following conditions: | ||
| + | |||
(a) The degree of bacteriological pollution of the water is moderate, reasonably uniform, and not imbedded in suspended solids, for example, within the bodies of worms; | (a) The degree of bacteriological pollution of the water is moderate, reasonably uniform, and not imbedded in suspended solids, for example, within the bodies of worms; | ||
| + | |||
(b) The turbidity and colour of the water do not exceed 5-10 units; | (b) The turbidity and colour of the water do not exceed 5-10 units; | ||
| + | |||
(c) The content of iron or manganese or both do not exceed 0.3 mg/L; and | (c) The content of iron or manganese or both do not exceed 0.3 mg/L; and | ||
| + | |||
(d) Taste- or odour-producing substances are absent or do not require chlorine doses that inevitably produce a chlorine taste in the treated water. | (d) Taste- or odour-producing substances are absent or do not require chlorine doses that inevitably produce a chlorine taste in the treated water. | ||
| + | |||
There is a contact period of at least 20 minutes between the point of chlorination and the first service connection supplied with the water. In cases where water is pumped directly from the source (e.g. well) into the distribution system, chlorine may be applied. | There is a contact period of at least 20 minutes between the point of chlorination and the first service connection supplied with the water. In cases where water is pumped directly from the source (e.g. well) into the distribution system, chlorine may be applied. | ||
| − | + | ||
| + | ===== Chlorination Guideline ===== | ||
| + | |||
(a) Chlorine solutions lose strength while standing or when exposed to air or sunlight. Make fresh solutions frequently to maintain the necessary residual. | (a) Chlorine solutions lose strength while standing or when exposed to air or sunlight. Make fresh solutions frequently to maintain the necessary residual. | ||
| + | |||
(b) Maintain a free chlorine residual of 0.3 mg/l after 30 minutes contact time. Residual chlorine should be measured every day. | (b) Maintain a free chlorine residual of 0.3 mg/l after 30 minutes contact time. Residual chlorine should be measured every day. | ||
| + | |||
(c) Once the chlorine dosage is increased to meet greater demand, do not decrease it unless the raw water quality improves. | (c) Once the chlorine dosage is increased to meet greater demand, do not decrease it unless the raw water quality improves. | ||
| + | |||
(d) When there is a risk of cholera or an outbreak has already occurred, maintain the chlorine residuals as follows: | (d) When there is a risk of cholera or an outbreak has already occurred, maintain the chlorine residuals as follows: | ||
| + | |||
(i) Distribution system: 0.5 mg/l | (i) Distribution system: 0.5 mg/l | ||
| + | |||
(ii) Tanker trucks at filling point: 2 mg/l | (ii) Tanker trucks at filling point: 2 mg/l | ||
| − | + | ||
| + | ===== Chlorination Methods ===== | ||
Disinfection is carried out by applying chlorine or chlorine compounds. The methods of application are as follows: | Disinfection is carried out by applying chlorine or chlorine compounds. The methods of application are as follows: | ||
| + | |||
(a) Preparing weak solution by bleaching powder, | (a) Preparing weak solution by bleaching powder, | ||
| + | |||
(b) Preparing weak solution by electrolysing brine solution, | (b) Preparing weak solution by electrolysing brine solution, | ||
| + | |||
(c) By adding chlorine either in the form of gas or solution prepared from dissolving chlorine gas in small feed of water. | (c) By adding chlorine either in the form of gas or solution prepared from dissolving chlorine gas in small feed of water. | ||
| − | + | ||
| + | ==== Disinfection by Bleaching Powder ==== | ||
| + | |||
Bleaching powder or calcium hypochlorite is a chlorinated lime, which contains about 25 to 34% of available chlorine by weight. Chlorine being a gas is unstable and as such it is mixed with lime to retain its strength for a longer period, as far as possible. The bleaching powder is hygroscopic in nature. It loses its chlorine strength rapidly due to poor storage and hence should not be stored for more than three months. The method of chlorination by bleaching powder is known as hypo-chlorination. The combined action of hypochlorous acid and hypochlorite ion brings about the disinfection of water. | Bleaching powder or calcium hypochlorite is a chlorinated lime, which contains about 25 to 34% of available chlorine by weight. Chlorine being a gas is unstable and as such it is mixed with lime to retain its strength for a longer period, as far as possible. The bleaching powder is hygroscopic in nature. It loses its chlorine strength rapidly due to poor storage and hence should not be stored for more than three months. The method of chlorination by bleaching powder is known as hypo-chlorination. The combined action of hypochlorous acid and hypochlorite ion brings about the disinfection of water. | ||
| − | + | ||
| + | ===== Preparation of Solution ===== | ||
| + | |||
The concentrated solution of bleaching powder is prepared in one or two tanks of capacity suitable for 24 hours requirement. The tank inside should be of glazed tiles or stoneware and should be covered. The tank should be under shade and not direct to sunlight. | The concentrated solution of bleaching powder is prepared in one or two tanks of capacity suitable for 24 hours requirement. The tank inside should be of glazed tiles or stoneware and should be covered. The tank should be under shade and not direct to sunlight. | ||
| + | |||
(a) The powder is first put on a perforated slab placed longitudinally inside the tank at a higher level, with respect to bed level of tank; | (a) The powder is first put on a perforated slab placed longitudinally inside the tank at a higher level, with respect to bed level of tank; | ||
| + | |||
(b) Water is sprinkled on the powder through a perforated pipe above this perforated slab; | (b) Water is sprinkled on the powder through a perforated pipe above this perforated slab; | ||
| + | |||
(c) The solution of bleaching powder and water now enters the tank. The solution is rotated for thorough mixing of powder with water by a hand driven/motor reduction gear operated slow speed stirrer is now ready for use as disinfectant; | (c) The solution of bleaching powder and water now enters the tank. The solution is rotated for thorough mixing of powder with water by a hand driven/motor reduction gear operated slow speed stirrer is now ready for use as disinfectant; | ||
| + | |||
(d) The precipitates of calcium hydroxide settle at the bottom of the tank. The supernatant water, which contains OCl, Cl- plays important role in disinfection; | (d) The precipitates of calcium hydroxide settle at the bottom of the tank. The supernatant water, which contains OCl, Cl- plays important role in disinfection; | ||
| + | |||
(e) For effectiveness of chlorination, contact period of at least 4 hours should be maintained. | (e) For effectiveness of chlorination, contact period of at least 4 hours should be maintained. | ||
| − | + | ||
| + | ===== Dosing of Solution ===== | ||
The solution is discharged to a small measuring tank at a lower level through PVC pipe or any other material resistant to chlorine. The level of water in this tank is maintained constant through a float valve. The solution is dosed to the clear water channel by gravity at the time of entry to clear water reservoir. The dose has to be monitored properly, depending on the desired residual chlorine required in clear water reservoir. The waste precipitates at the bottom of tanks are taken out occasionally by scour valve. | The solution is discharged to a small measuring tank at a lower level through PVC pipe or any other material resistant to chlorine. The level of water in this tank is maintained constant through a float valve. The solution is dosed to the clear water channel by gravity at the time of entry to clear water reservoir. The dose has to be monitored properly, depending on the desired residual chlorine required in clear water reservoir. The waste precipitates at the bottom of tanks are taken out occasionally by scour valve. | ||
| − | + | ||
| + | ===== Safety precautions ===== | ||
| + | |||
(a) The operating personnel should use hand gloves, aprons and other protective apparel, while handling and mixing; | (a) The operating personnel should use hand gloves, aprons and other protective apparel, while handling and mixing; | ||
| + | |||
(b) The valves, stirrer, tanks, plumbing arrangements require renovation at every 6 months or so. | (b) The valves, stirrer, tanks, plumbing arrangements require renovation at every 6 months or so. | ||
| − | + | ||
| + | ==== Chlorination by Gaseous Chlorine ==== | ||
Elemental chlorine at a normal pressure is a toxic, yellow green gas, and is liquid at high pressure. Chlorine gas is released from a liquid chlorine cylinder by a pressure reducing and flow control valve operating at a pressure less than atmospheric pressure. The gas is injected in the water supply pipe where highly pressurized water is passed through a venture creating a vacuum that draws the chlorine in to the water stream. Adequate mixing and contact time must be provided after injection to ensure complete disinfection of pathogens. It may be necessary to control the water pH. A basic system consists of chlorine cylinder mounted with vacuum regulator, chlorine gas injectors, and a contact tank or pipe. Prudence or state regulation would require that a second cylinder and gas regulator be provided with a changeover valve to ensure continuity of disinfection. Additional safety and control system may be required. | Elemental chlorine at a normal pressure is a toxic, yellow green gas, and is liquid at high pressure. Chlorine gas is released from a liquid chlorine cylinder by a pressure reducing and flow control valve operating at a pressure less than atmospheric pressure. The gas is injected in the water supply pipe where highly pressurized water is passed through a venture creating a vacuum that draws the chlorine in to the water stream. Adequate mixing and contact time must be provided after injection to ensure complete disinfection of pathogens. It may be necessary to control the water pH. A basic system consists of chlorine cylinder mounted with vacuum regulator, chlorine gas injectors, and a contact tank or pipe. Prudence or state regulation would require that a second cylinder and gas regulator be provided with a changeover valve to ensure continuity of disinfection. Additional safety and control system may be required. | ||
| Line 789: | Line 1,022: | ||
Chlorine is very effective for removing almost all pathogen and is appropriate for both a primary and secondary disinfectant. The limitation with this is it is dangerous gas that is lethal at concentrations as low as 0.1 per cent air by volume. | Chlorine is very effective for removing almost all pathogen and is appropriate for both a primary and secondary disinfectant. The limitation with this is it is dangerous gas that is lethal at concentrations as low as 0.1 per cent air by volume. | ||
| − | + | ||
| + | ===== Working Safely around Chlorine Gas ===== | ||
Any water utility that uses chlorine should have written procedures for its chlorine system operation. Even the use of powdered chlorine should have written procedures. | Any water utility that uses chlorine should have written procedures for its chlorine system operation. Even the use of powdered chlorine should have written procedures. | ||
Before starting any chlorination process, the following precautions should be taken: | Before starting any chlorination process, the following precautions should be taken: | ||
| + | |||
(a) If a faucet with good flowing water is not available close by, make ready a 5- gallon container of fresh water, but make sure it is away from the chlorine cylinder or storage area. This is to ensure that if the chlorine accidentally comes in contact with your eyes or skin, you can flush the affected areas with copious amounts of fresh water for at least 10-15 minutes, | (a) If a faucet with good flowing water is not available close by, make ready a 5- gallon container of fresh water, but make sure it is away from the chlorine cylinder or storage area. This is to ensure that if the chlorine accidentally comes in contact with your eyes or skin, you can flush the affected areas with copious amounts of fresh water for at least 10-15 minutes, | ||
| + | |||
(b) Flush the chlorine out. Do not just soak the affected surface. If you get some of the chlorine solution in your eyes, flush it out and immediately see your doctor, | (b) Flush the chlorine out. Do not just soak the affected surface. If you get some of the chlorine solution in your eyes, flush it out and immediately see your doctor, | ||
| + | |||
(c) Wear the prescribed safety clothing and equipment, specifically: | (c) Wear the prescribed safety clothing and equipment, specifically: | ||
| + | |||
(i) Goggles to protect your eyes from contact with the chlorine in any form, | (i) Goggles to protect your eyes from contact with the chlorine in any form, | ||
| + | |||
(ii) Rubber gloves and rubber boots certified for use around the chemical to protect your hands and feet, | (ii) Rubber gloves and rubber boots certified for use around the chemical to protect your hands and feet, | ||
| + | |||
(iii) Waterproof suit, coveralls or a full-length apron. | (iii) Waterproof suit, coveralls or a full-length apron. | ||
| − | + | ||
| + | ===== Housekeeping/Chlorine Storage ===== | ||
| + | |||
(a) Use signs to clearly identify all areas where chlorine is used or stored. Only qualified personnel should be permitted to enter these areas, | (a) Use signs to clearly identify all areas where chlorine is used or stored. Only qualified personnel should be permitted to enter these areas, | ||
| + | |||
(b) Do not store materials that may react violently with chlorine in the same room as chlorine. Put up visible warning signs prohibiting persons from taking these materials where the chlorine is stored, | (b) Do not store materials that may react violently with chlorine in the same room as chlorine. Put up visible warning signs prohibiting persons from taking these materials where the chlorine is stored, | ||
| + | |||
(c) Do not store chlorine near busy roadways or where vehicles operate. Chlorine reacts with carbon monoxide to produce phosgene, an extremely poisonous gas, | (c) Do not store chlorine near busy roadways or where vehicles operate. Chlorine reacts with carbon monoxide to produce phosgene, an extremely poisonous gas, | ||
| + | |||
(d) Store chlorine cylinders and containers in a cool, dry, and relatively isolated area, protected from weather and extreme temperatures: | (d) Store chlorine cylinders and containers in a cool, dry, and relatively isolated area, protected from weather and extreme temperatures: | ||
| + | |||
(i) When storing cylinders and containers outside, shield them from direct sunlight, | (i) When storing cylinders and containers outside, shield them from direct sunlight, | ||
| + | |||
(ii) When storing chlorine containers inside, store the containers in a well- ventilated building, away from any heat sources. | (ii) When storing chlorine containers inside, store the containers in a well- ventilated building, away from any heat sources. | ||
(e) Use cylinders and containers on a “First-In, First-Out” basis, | (e) Use cylinders and containers on a “First-In, First-Out” basis, | ||
| + | |||
(f) Clearly tag or mark empty cylinders and separate them from full cylinders, | (f) Clearly tag or mark empty cylinders and separate them from full cylinders, | ||
| + | |||
(g) Determine the most appropriate location for emergency equipment. Emergency equipment and a faucet should be available in a readily accessible location, but not inside the chlorine room because a worker (and emergency response staff) trying to use the emergency equipment or faucet during a chlorine leak risks further exposure, | (g) Determine the most appropriate location for emergency equipment. Emergency equipment and a faucet should be available in a readily accessible location, but not inside the chlorine room because a worker (and emergency response staff) trying to use the emergency equipment or faucet during a chlorine leak risks further exposure, | ||
| + | |||
(h) Store cylinders upright and secure them against tipping over and rough handling. Cylinders will discharge vapour when upright and discharge liquid when upside-down. Since chlorine gas tends to sink, provision should be made for low-placed ventilation near the floor that allows it to dissipate outward, as well as high-placed ventilation that allows the chlorine mist (the gas mixed with air) which tends to go upward, also to dissipate. | (h) Store cylinders upright and secure them against tipping over and rough handling. Cylinders will discharge vapour when upright and discharge liquid when upside-down. Since chlorine gas tends to sink, provision should be made for low-placed ventilation near the floor that allows it to dissipate outward, as well as high-placed ventilation that allows the chlorine mist (the gas mixed with air) which tends to go upward, also to dissipate. | ||
| − | + | ===== Handling Chlorine Cylinders ===== | |
(a) Handle containers with care while moving or storing them. Do not drop or allow containers to strike objects, | (a) Handle containers with care while moving or storing them. Do not drop or allow containers to strike objects, | ||
| + | |||
(b) Use new gaskets as recommended by the chlorine supplier each time a cylinder or container is connected, | (b) Use new gaskets as recommended by the chlorine supplier each time a cylinder or container is connected, | ||
| + | |||
(c) Follow the chlorine supplier’s recommended disposal procedures for leaking containers. Do not modify, alter, or repair containers and valves. Only the supplier should carry out these tasks, | (c) Follow the chlorine supplier’s recommended disposal procedures for leaking containers. Do not modify, alter, or repair containers and valves. Only the supplier should carry out these tasks, | ||
| + | |||
(d) Ensure that cylinders have valve protection hoods in place when not connected to a system, | (d) Ensure that cylinders have valve protection hoods in place when not connected to a system, | ||
| + | |||
(e) Do not lift a cylinder by its valve protection hood. The hood is not designed to carry the weight of a cylinder, | (e) Do not lift a cylinder by its valve protection hood. The hood is not designed to carry the weight of a cylinder, | ||
| + | |||
(f) If possible, open valves by applying a steady force to a 200 mm (8 in) wrench, without applying an impact force and without using an extension on the wrench. If this does not work, apply a light impact force by smacking the wrench with the heel of your hand, | (f) If possible, open valves by applying a steady force to a 200 mm (8 in) wrench, without applying an impact force and without using an extension on the wrench. If this does not work, apply a light impact force by smacking the wrench with the heel of your hand, | ||
| + | |||
(g) Do not use a wrench longer than 200 mm (8 in) to open or close valves. To prevent valve damage that could cause leaks do not use tools such as pipe wrenches or hammers. Valves on cylinders are designed to deliver full volume after one complete counter clockwise turn. Valves may be damaged if turned beyond this point. Immediately return containers with damaged or inoperable (but not leaking) valves to the supplier, | (g) Do not use a wrench longer than 200 mm (8 in) to open or close valves. To prevent valve damage that could cause leaks do not use tools such as pipe wrenches or hammers. Valves on cylinders are designed to deliver full volume after one complete counter clockwise turn. Valves may be damaged if turned beyond this point. Immediately return containers with damaged or inoperable (but not leaking) valves to the supplier, | ||
| + | |||
(h) If the valve is very difficult to open, loosen the packing nut slightly. Tighten the packing nut after the valve is opened or closed. | (h) If the valve is very difficult to open, loosen the packing nut slightly. Tighten the packing nut after the valve is opened or closed. | ||
| Line 829: | Line 1,086: | ||
| − | + | ==== Electro-chlorinator ==== | |
Chlorine is instantly produced by electrolyzing brine solution. Common salt is mixed with water to prepare brine solution. This solution is passed through an Electrolyser of electrodes comprising of anodes and cathodes, which are energised by D.C. current to produce NaOCl. This solution of sodium hypo chlorite is used as disinfectant. Basically, the electro chlorinator set comprises of two compartments; one comprising of brine solution tank, electrolyser, cooler, etc. and the other comprising of compact panel board (rectifier). Normal life of electro chlorinator is 12 years provided reconditioning of the electrodes at regular interval of four years is carried out. | Chlorine is instantly produced by electrolyzing brine solution. Common salt is mixed with water to prepare brine solution. This solution is passed through an Electrolyser of electrodes comprising of anodes and cathodes, which are energised by D.C. current to produce NaOCl. This solution of sodium hypo chlorite is used as disinfectant. Basically, the electro chlorinator set comprises of two compartments; one comprising of brine solution tank, electrolyser, cooler, etc. and the other comprising of compact panel board (rectifier). Normal life of electro chlorinator is 12 years provided reconditioning of the electrodes at regular interval of four years is carried out. | ||
| − | + | ||
| + | ==== Other Disinfectants ==== | ||
The other chemical based disinfectants generally in use are ionized silver coating, gaseous chlorine, ozone, chloramine, potassium permanganate and hydrogen peroxide. Alternatives to chemical disinfection, such as UV irradiation, are also being used for disinfection of drinking water. | The other chemical based disinfectants generally in use are ionized silver coating, gaseous chlorine, ozone, chloramine, potassium permanganate and hydrogen peroxide. Alternatives to chemical disinfection, such as UV irradiation, are also being used for disinfection of drinking water. | ||
| − | + | ==== Ozonation ==== | |
Ozone is very strong oxidiser and powerful disinfecting property. Avery small concentration of ozone in water makes it free from bacteria, virus and pathogen much faster and with lesser concentration in a most effective manner. Ozone, an allotrope of oxygen having three atoms to each molecule, is a powerful oxidizing and disinfecting agent. It is formed by passing dry air through a system of high voltage electrodes. The major elements of an ozonation system are: | Ozone is very strong oxidiser and powerful disinfecting property. Avery small concentration of ozone in water makes it free from bacteria, virus and pathogen much faster and with lesser concentration in a most effective manner. Ozone, an allotrope of oxygen having three atoms to each molecule, is a powerful oxidizing and disinfecting agent. It is formed by passing dry air through a system of high voltage electrodes. The major elements of an ozonation system are: | ||
| + | |||
(a) Air preparation of oxygen feed, | (a) Air preparation of oxygen feed, | ||
| + | |||
(b) Electrical power supply, | (b) Electrical power supply, | ||
| + | |||
(c) Ozone generation usually using a corona discharge cell consisting of two electrodes, | (c) Ozone generation usually using a corona discharge cell consisting of two electrodes, | ||
| + | |||
(d) Ozone context chamber, and | (d) Ozone context chamber, and | ||
| + | |||
(e) Ozone exhausts gas destruction. | (e) Ozone exhausts gas destruction. | ||
| + | |||
Advantages of ozonation system is that it requires shorter contact time and doses than chlorine, ozone does not directly produce halogenated organic materials unless a bromide ion is present. Limitations of ozonation system, ozone gas is unstable and must be generated onsite. A secondary disinfectant, usually chlorine, is required because ozone does not maintain an adequate residual in water. | Advantages of ozonation system is that it requires shorter contact time and doses than chlorine, ozone does not directly produce halogenated organic materials unless a bromide ion is present. Limitations of ozonation system, ozone gas is unstable and must be generated onsite. A secondary disinfectant, usually chlorine, is required because ozone does not maintain an adequate residual in water. | ||
| − | + | ==== Operation and Maintenance for Ultra-filtration, Micro filtration and Nano filtration ==== | |
Basically, ultrafiltration and microfiltration are the protection mechanism of nanofiltration and these should precede Nano filtration, for more details refer Volume I. Because application of membrane filtration technology is still rare in Tanzania at the moment, the current edition of the manual refers designers to the manufacturers of membrane filters for all design specifications sample websites of the manufacturers are provided at the end of this DCOM Manual . | Basically, ultrafiltration and microfiltration are the protection mechanism of nanofiltration and these should precede Nano filtration, for more details refer Volume I. Because application of membrane filtration technology is still rare in Tanzania at the moment, the current edition of the manual refers designers to the manufacturers of membrane filters for all design specifications sample websites of the manufacturers are provided at the end of this DCOM Manual . | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | Previous Page: [[Part_C|Part C: Operation and Maintenance of Water Treatment]] << >> Next Page: [[Chapter Thirteen: Treatment for Special Water Sources]] | ||
| + | |||
| + | </div> | ||
Latest revision as of 13:34, 20 July 2022
Contents
- 1 Chapter Twelve: Water Treatment
- 1.1 Pre-treatment
- 1.2 Primary Treatment
- 1.3 Secondary Treatment
- 1.3.1 Clarification
- 1.3.2 Coagulation and Flocculation
- 1.3.2.1 Coagulation
- 1.3.2.2 Flocculation
- 1.3.2.3 Rapid Sand Filtration Plant
- 1.3.2.4 Sedimentation
- 1.3.2.5 Operation and Maintenance for Defluoridation
- 1.3.3 Tertiary Treatment
1 Chapter Twelve: Water Treatment
The principal objective of water treatment in water supply industry is to produce water that is fit for domestic use from a raw water source throughout the water supply system to the consumers. The raw water available from sources particularly surface water sources is normally not suitable for drinking purposes. Thus, raw water needs treatment to produce safe and potable drinking water. Some of the common treatment processes of the conventional treatment facilities include the following:
(a) Pre-treatment – Scum and floating matters removal, Screening (fine and coarse), Sand trap, Grit removal, Pre-chlorination,
(b) Primary treatment – Sedimentation, Primary filtration, Floatation, Aeration.
(c) Secondary treatment – Coagulation, Flocculation, Clarification, Filtration, Softening, Reverse Osmosis, Capacitive De-Ionisation (CDI), Ion Exchanger, De-fluoridation, Adsorption, Constructed wetlands.
(d) Tertiary treatment – Disinfection, Softening, Utra-filtration, Microfiltration, Nano-filtration, Water conditioning, Water polishing.
1.1 Pre-treatment
1.1.1 Scum and Floating Materials Skimmer
This is the unit operation that enables the manual or automated removal of the scum and floating matter ahead of the screening units. These are designed to skim the entire width of the approach area ahead of the screens. It is one of the most popular styles of scum skimmers used is the scum pipe. It is used in 90%-95% of applications. The other 5% of the time when a skimmer is needed, a helical or paddle wheel skimmer is used. The removal of scum during clarification is an essential step to decrease organic loadings and scum build up in subsequent treatment processes.
The rotating scum troughs and skimmers efficiently remove all grease, scum and floating material from the water surface in rectangular clarifiers and settling basins. Engineered for both corrosion-resistance and strength, the Scum removal systems are available in non-metallic or metallic designs. The scum skimming blade directs the scum through the opening and into contact with the helical blade, and the helical blade is rotated by the drive unit in a manner tending to keep the scum in the trough while moving the scum lengthwise along the trough to a discharge point outside the tank. In a further embodiment, the trough contains a sloped portion rising above the liquid level to allow the liquids to drain from the scum being transported (Tank Enviro Systems website: https://www.tankenviro.com.au/products/scum-removal).
1.1.2 Screening/Straining
This unit operation consists of fine screens and coarse screens which perform the task of removal of all fine and coarse materials that may block the screen or damage downstream appurtenances or machines. This is a physical, pre-treatment process used to remove weeds, grass, twigs, bilharzial snails and other freshwater crustacean as well as coarser particles including plastics, tins and others so that they do not enter the pumping, treatment, or supply system. Screens are placed at the entrance to the intake of a water supply project
1.1.3 Grit Removal Channels
The purposes of grit channels in the water and wastewater treatment plants are as follows:
- To protect pumps and other mechanical parts from excessive wear and tear,
- To avoid undue clogging/filling up of subsequent unit operations,
- To differentially remove grit but not the organic particulates in water.
Grit channels are used for peak flow. They should be used either one at a time, or every day. Grit channels should be cleaned every day. Proper and efficient removal of silt in grit channels will improve the functioning of treatment.
1.1.4 Sand Traps
This pre-treatment unit is designed to trap sand after water has been guided into the intake chamber in order to reduce the potential for wear and tear as well as silting up the unit operations that are located downstream of the intake structure. The minimum diameter of such sand traps is 75 mm and the bigger the main intake pipe, the bigger is the flushing pipe for sand.
1.1.5 Pre-chlorination
This is a unit operation that is used for purposes of controlling algae growth in raw water and the process of preparation of the chemical to facilitate dosing should include the standard preparation of the aqueous solution as done during disinfection. This is often added upon establishment of occurrence of algal blooms during certain periods of the year as confirmed by laboratory tests undertaken daily. The amount of the dose should be established daily in order to pre-determine the pre-chlorination dose that has to ensure no interference with downstream unit operations in case the flow sheet involves biological treatment processes like slow sand filter or others.
1.1.6 Pre-Sedimentation Unit
Pre-sedimentation is the removal of coarse suspended matter (such as grit) depending merely on gravity. This type of sedimentation typically takes place in a reservoir, grit basin, debris dam, or sand trap at the beginning of the treatment process. The pre-sedimentation removes most of the sediment from the water at the pre-treatment stage and it reduces the load on the coagulation/flocculation basin and on the sedimentation chamber, as well as reducing the volume of coagulant chemicals required to treat the water.
For the treatment of highly turbid raw water during the rainy season, solids loadings including larger particles decreased substantially with the application of Pre-sedimentation in the water treatment plant during the rainy season (Kwak et al., 2010). Contaminants from raw water could be removed step-by-step following sequential treatment processes. The selection and arrangement of different treatment processes are of great importance for achieving high contaminant removal efficiency. Pre-sedimentation has various effects on water treatment plant operation, and the produced water depends on raw water quality. (Source:https://www.researchgate.net/publication/323643034_Pre-sedimentation_tank_effects_on_water_treatment_unit_operation)
1.1.7 Tube Settlers
A set of small diameter tubes (inclined about 60°) having a large wetted perimeter relatively to wetted area when introduced in conventional sedimentation tank, provide laminar flow condition and with low surface loading rate yield good settlement of solids. The tubes which are inclined at about 60 degree found to yield good results. The tubes may be square, circular, hexagonal, diamond shaped, triangular, rectangular shaped. In most of the sedimentation tank the shape will be of thin sheets. There is also another type of settlers widely known as Lamella settlers.
1.1.8 Water Pre-conditioning
Water pre-conditioning can entail a number of pre-treatments undertaken prior to pre-chlorination which is often the final step in pre-treatment. This unit operation involves adjustment of the pH upstream in order to ensure the chemicals used during further treatment processes are dosed to water that has the correct pH range for maximum efficiency.
1.2 Primary Treatment
1.2.1 Sedimentation
Sedimentation is a physical water treatment process using gravity to remove suspended solids from water. Solid particles entrained by the turbulence of moving water may be removed naturally by sedimentation in the still water of lakes and oceans. Settling basins are ponds constructed for the purpose of removing entrained solids by sedimentation. Clarifiers are tanks built with mechanical means for continuous removal of solids being deposited by sedimentation.
Sedimentation tank is used as a component of a modern system of water supply or wastewater treatment. It allows suspended particles to settle out of water or wastewater as it flows slowly through the tank by gravity, thereby providing some degree of purification. Suspended materials may be particles, such as clay or silts, originally present in the source water.
1.2.2 Lamella Plate Settlers (Inclined Plate Settlers)
A lamella clarifier or inclined plate settler (IPS) is a type of settler designed to remove particulates from liquids. They are often employed in primary water treatment in place of conventional settling tanks. They are commonly used in industrial water treatment. Unlike conventional clarifiers they use a series of inclined plates. These inclined plates provide a large effective settling area for a small footprint. The inlet stream is stilled upon entry into the clarifier. Solid particles begin to settle on the plates and begin to accumulate in collection hoppers at the bottom of the clarifier unit. The sludge is drawn off at the bottom of the hoppers and the clarified liquid exits the unit at the top over a weir.
Lamella clarifiers can be used in a range of industries including mining and metal finishing, as well as used to treat groundwater, industrial process water and backwash from sand filters. Lamella clarifiers are ideal for applications where the solids loading are variable and solids sizing is fine and are more common than conventional clarifiers at many industrial sites due to their smaller footprint. Lamella clarifiers are also used in the municipal wastewater treatment processes (https://en.wikipedia.org/wiki/Lamella_clarifier).
1.2.2.1 Operation and Maintenance of Lamella Settlers
Properly designed and constructed Lamella plate settlers require minimal operator attention. However, provisions for access and the maintenance should be considered. Access Lamella plate settlers basically require little maintenance. Access walk ways over the basin area if not provided already needs to be provided during maintenance depending upon the requirement of the maintaining agency.
1.2.2.2 Maintenance
Lamella plate equipment sedimentation basin does not require any adjustment by the operating staff. Normal maintenance is dependent on the materials selected for construction. Periodic disassembly of the plate pack system is recommended if painted carbon steel equipment is used. Stainless steel construction however minimizes routine maintenance. If process upset such as coagulant over dose or biological growth occurs, the basin may have to be drained and or plate cleaned with high pressure hose.
1.2.2.3 Record Keeping
Daily operations log of process performance and water quality characteristics should:
(a) Record inflow and outflow turbidity and inflow temperature,
(b) Process production inventory (amount of water processed and volume of sludge produced),
(c) Process equipment performance (type of equipment in operation, maintenance procedures performed and equipment calibration).
1.2.2.4 Cleaning and Maintenance of Lamella Clarifiers
It is important to carry out cleaning the Lamella Plate Settlers in order to improve the performance of the lamellar modules and ensure a greater longevity of the installation.
(a) Emptying procedure before cleaning
(i) While the decanter is still filled with water, start spraying the surface of the lamellar module with pressurized water: pressure should not exceed 6 to 8 bar. Modules should be washed on an ongoing basis. Therefore, it is recommended to have more than 1 worker carrying out the cleaning. In order to water down the lamellas correctly, it is recommended that the maintenance workers walk on the surface of the lamellar modules using wood. In this way, modules are prevented from breaking. These ruptures do not influence performance but they affect the visual aspect of the installation. As the surface of the lamellar module is being sprayed, the water level in the clarifier must descend progressively, especially while the descent affects the length/height of the modules. Close the valves (for short intervals of time) to ensure homogeneous washing. This will dilute any organic matter deposited/adhered on the walls of the pipes and avoid its drying that could reduce the particle slippage, thus impairing the effectiveness of the process;
(ii) During the emptying of the clarifier, don’t stop spraying the water from the surface downwards, and keep the scraper in operation and the sludge purge pumping because the process tends to produce a lot of solid sedimentation. Perfect sludge collection will ensure greater lamellar performance;
(iii) Once the clarifier has been emptied, proceed to internal inspection of the equipment. To enter inside the clarifier, you can remove one of the lamellar packs to place a staircase or any other appropriate element to help you go down. It is usually necessary to disarm part of the Ant-Flotation System (AFS) to be able to remove the module;
(iv) It is important to clean rain gutter channels, especially if they are tubular and with holes.
(b) Parts of the Lamella Settler/Clarifier that need reviewing
(i) Supporting structure review: if the structure is iron-made, check for any sign of corrosion or degradation;
(ii) Review the structure-supporting brackets to ensure that the profiles are correctly fastened to the walls of the clarifier;
(iii) Make sure that the lamellas are properly leaning on the supporting structure;
(iv) Find out if any of the areas in the lamellar module are still clogged with sludge. Should it be the case, make sure to clean them insistently as they will be the most prone to mud accumulation, which can affect the supporting structure;
(v) Review the bottom scraper, its state, the wear of wheels or skates, the state of the concrete. Find out if any replacement is needed.
(c) Recommendations
(i) Before entering the clarifier for inspection purposes, we recommend to make sure that the supporting beams of the lamellar modules have not yielded: sometimes when designing the support structure, only the weight of the lamellar module is taken into account, leaving aside the weight of the sludge. But 1m3 of lamellas, when empty, may weigh 50 kg, however, with 100% sludge, it can weigh up to 1,300 kg. For safety reasons, it is recommended to check the supporting structure before entering the clarifier;
(ii) The plant operators know their sludge production and will safely determine when the clarifiers need cleaning. However, it is recommended to carry out maintenance at least 1-2 times a year;
(iii) Once the clarifier is clean, it is absolutely necessary to refill it with water. Otherwise, continuous and long-term exposure to the sun may alter the molecular chain of raw materials, causing damages or deformations on the medium term;
(iv) Even if lamellas present a constant thickness of 1 mm, they are protected against UV and welded with our system of reinforcement by points, it is advisable to strictly respect the previous recommendation;
(v) Should the modules need to be left outdoors without water for longer periods of time, it is recommended to cover them with a tarpaulin to avoid direct contact with the sun;
(vi) Please consider that a lamella clarifier produces approximately 4 times more sludge than a clarifier without lamellar modules. Based on this, it is indispensable to equip the clarifier with a perfect lower sludge extraction system to prevent it from collapsing and avoid the sludge to invade clean water collection channels.
1.2.3 Slow Sand Filtration Plant
A Slow Sand Filter Plant consists of a box which is rectangular or circular in shape made either of concrete or masonry. This box is normally one component in a treatment process which may involve preliminary settlement of solids and / or roughing filters and post chlorination. Typically the slow sand filter plant consists of two rectangular operating in parallel, one filter unit is kept in operation and other for maintenance. The filter units also comprise pipe fittings, under drains and graded gravel to support the filter’s sand bed. A flow indicator is used for checking the flow rate. The turbidity of the inlet water is checked to ensure the water is of an acceptable turbidity to prevent rapid blocking of the filter. Turbidity is also measured at the outlet to check the filter is functioning properly. The supervising manager carries out daily bacteriological tests on the filtered water.
1.2.3.1 Operation and Maintenance of Slow Sand Filter
(a) Daily activities
(i) Check the rate of filtration on the flow indicator – adjust the rate of filtration as needed by turning the filtered water valve;
(ii) Check the water level in the filter – adjust the inlet vale as needed to maintain a constant water level;
(iii) Remove scum and floating material by further opening the inlet valve for short time;
(iv) Check the water level in the clear well;
(v) Sample and check water turbidity – if the inflow turbidity is too high close the intake; if the outflow turbidity is too high report to the supervisor;
(vi) Testing water quality;
(vii) Complete the log book;
(viii) Testing Water Quality: Daily monitoring of water quality may be done whether it is slow sand filter or rapid sand filter. If the water supply scheme is having laboratory at the water treatment plant site, water quality testing both the raw water and treated water may be carried out daily.
(b) Weekly activities
Clean the water treatment plant site.
(c) Monthly activities
(i) Shut down the filter unit – remove scum and floating material;
(ii) Brush the filter walls; close the inlet, filtered water and distribution valves;
(iii) Drain water to 20 cm below the sand level;
(iv) Increase the filtration rate in the other filter to 0.2 m/h;
(v) Clean the drained down filter bed – wash boots and equipment before use; scrape upper 2-3 cm in narrow strips and remove scrapings from filter;
(vi) Check, and service, exposed inlet and drain valves; remove cleaning equipment and level sand surface; check and record depth of sand bed;
(vii) Adjust inlet box to the new sand level;
(viii) Re-start the filter – open the recharge valve; check sand surface and level as needed;
(ix) When water is 20 cm above the sand, open the inlet valve;
(x) Open the filtered water valve and stop when filtration rate reaches 0.02 m/h;
(xi) Open waste valve for outflow water to flow to waste;
(xii) Open filtered water valve to increase filtration rate every hour by 0.02 m/h until a rate of 0.1 m/h is reached;
(xiii) Adjust and check flow daily until safe to drink;
(xiv) Close waste valve and open distribution valve to pass filtered water into the supply;
(xv) Decrease filtration rate of other filter to 0.1 m/h;
(xvi) Wash the filter scrapings and store the clean sand.
(d) Quarterly activities - cleaning of filter
(i) Close the water inlet and allow the filter to discharge clear water for at least 8-10 hours;
(ii) Close the treated water outlet valve;
(iii) Open the waste water outlet till the water in the filter bed reaches up to 0.1-0.2 mm from bottom;
(iv) Remove wastage on top of the filter;
(v) Remove the sand as little as possible, not more than 20-30 mm (the Schmutzdecke). Wastage can be removed manually or with mechanical equipment. Care should be taken to avoid any contamination while removal of waste in the filter tank by observing hygiene and cleaning it as quickly as possible;
(vi) Level the sand in the filter;
(vii) Re-start the filter by opening inlet valves and outlet valves.
After sand cleaning is done for 20-30 times, the depth of sand layer will decrease and needs to replace.
(e) Annual activities
(i) Check if filter is water tight: close all valves and fill filter box from inlet valve until it overflows – close valve;
(ii) leave for 24 hours and check if water level reduces; if filter box leaks, report for repair;
(iii) open filtered water valve to fill outlet chamber and when full, close valve; leave for 24 hours and check if water level reduces; if chamber leaks, report for repair;
(iv) open drain valve to empty filter; clean the clear well in the outlet chamber;
(v) restart filter as per the monthly clean plan.
(f) Every two – three years, activities
(i) Re-sand the filter units – clean the filter as in a monthly filter clean;
(ii) open drain valve to empty water from the sand bed;
(iii) remove strip of old sand to one side;
(iv) place new clean sand on top of exposed gravel, and level;
(v) place old sand on top of the new sand to the correct depth of 0.8 m in total, and level the surface;
(vi) continue in strips until filter is re-sanded; adjust inlet box to new sand level;
(vii) Re-start the filter as per the monthly clean plan.
(g) Random checks
Checks on the functioning of the plant by supervising staff including turbidity tests through a turbidity meter, and bacteriological tests of the filtered water.
(h) Record keeping
Records have to be kept for the following activities:
(i) Daily Source water quality,
(ii) Daily Treated water quality,
(iii) Names of chemicals used,
(iv) Rates of feedings of chemicals,
(v) Daily consumption of chemical and quality of water treated,
(vi) Dates of cleaning of filter feds, sedimentation tank and clear water reservoir,
(vii) The date and hour of return to full service (end of re-ripening period),
(viii) Raw and filtered water levels (measured each day at the same hour) and daily loss of head,
(ix) The filtration rate, the hourly variations, if any,
(x) The quality of raw water in physical terms (turbidity, colour) and bacteriological terms (total bacterial count, E.Coli.) determined by samples taken each day at the same hour,
(xi) The same quality factors of the filtered water,
(xii) Any incidents occurring e.g. plankton development, rising Schmutzdecke, and unusual weather conditions,
(xiii) Precautions must be taken to minimize the chances of pollution of the filter bed surface by the labourers themselves.
1.2.3.2 Re-Sanding
Re-sanding becomes necessary when the depth of the sand bed drops to its minimum designed level (usually about 0.5 – 0.8 m above the supporting gravel, depending on the grain size of the filter sand/medium). This depth is usually indicated by a marker (such as a concrete block or a step in the filter box wall) set in the structure during the original construction to serve as an indication that this level has been reached and that sanding has become due. After scraping, add new clean sand up to a level shown in Figure 12.1 and place back the old sand that was scraped off the top. The old sand will reduce the number of days needed for ripening the filter.
Figure 12. 1: Details of Cleaning and Re-sanding of the SSF (Source: World Bank, 2012)
1.2.4 Roughing Filters
Operation of Roughing Filter Unit
Roughing Filter (RF) can easily be operated and maintained by trained local operators/technician/artisans. It does not depend on external inputs provided the necessary materials and tools are available. The daily activities of the caretaker are preferably supported by occasional visits of a supervisor attached to the operation and maintenance section of the governmental institution responsible for the water supply system. Important maintenance work should be carried out at the time when village participation can be involved. This is of particular importance with regard to manual cleaning of the RF.
Flow Pattern: For operational and economic reasons, it is recommended to continuously operate a RF-SSF plant at constant filtration rates for 24 hours/day. In case of a pumped Scheme, a raw water balancing tank is required. Removal of the coarse solids is a positive side effect of such a tank.
1.2.4.1 Roughing Filter Cleaning
Filter efficiency is not constant but may increase at the start of filter operation and certainly decreases when solid matter accumulates excessively in the filter. Hence, periodic removal of this accumulated matter restores filter efficiency and keeps the filter in good running condition. Hence, periodic removal of this accumulated matter is required to restore efficiency and possibly hydraulic filter performance. Filters are cleaned either hydraulically or manually, and the cleaning methods are dependent on the way solids accumulate in the filter. Hence, the cleaning procedures will therefore have to be adapted to the different filters.
1.2.4.2 Roughing Filter Maintenance
Major incidents are often the result of minor causes. This saying also applies to roughing filter maintenance. Filter maintenance is not very demanding as the pre-filters do not include any mechanical parts apart from the valves. Nevertheless, maintenance should aim at maintaining the plant in good condition right from the beginning. External assistance for maintenance work can usually be avoided if the following work is carried out properly by the local operator:
(a) periodic upkeep of the treatment plant's premise (grass cutting; removal of small bushes and trees which could impair the structures by their roots; removal of refuse);
(b) soil protection against erosion (especially surface water intake structures, the wash water drainage channels and surface runoff);
(c) repairing fissures in the walls of the different structures and replacing the chipped plaster;
(d) application of anti-corrosive agents to exposed metal parts (V-notch weirs, gauging rods, pipes);
(e) checking the different valves and drainage systems, and occasionally lubricating their moving parts;
(f) weeding the filter material;
(g) skimming off floating material from the free water table;
(h) washing out coarse settled material (distribution and inlet boxes);
(i) controlling the ancillaries and replacing defective parts (tools and testing equipment).
The term "periodic" does not only apply to the first point in this check list but to all of them. Proper maintenance of the treatment plant guarantees long-term use of the installations at low running costs.
1.2.5 Bank Filtration
Bank filtration is a water treatment technology that consists of extracting water from rivers by pumping wells located in the adjacent alluvial aquifer. During the underground passage, a series of physical, chemical, and biological processes take place, improving the quality of the surface water, substituting or reducing conventional drinking water treatment.
The efficiency of BF depends on local conditions including the hydrology and hydrogeology of the site, the geochemistry of water (from both the river and the aquifer), the geochemistry of microbial populations, and associated metabolic activity. This is the reason why it is difficult to define general procedures for identifying appropriate sites to implement the BF technique, as well as the expected efficiency of the process.
Optimal BF cleaning frequency
In general, the typical cleaning frequency is 7-8 years. Based on practical knowledge, the cycle cannot exceed ten years. As this deterioration of the filter layer is a function of the well operation (velocity of the bank filtrate, volume of produced water), a production load-based cleaning cycle.
1.2.6 Floatation Plant
A Dissolved Air Flotation (DAF) system creates microscopic air bubbles that are attached to incoming raw water and wastewater particles in order to float them. Once floated, they are separated from the raw water wastewater and skimmed from the top and into the float scum chamber. The treated water and wastewater then exits from near the bottom of the DAF. The DAF creates air bubbles with a sub-system called a Recycle Air Dissolving (RAD) system. Proper operation of the RAD system is key to DAF performance. The standard configuration RAD system is designed to take treated effluent from the DAF effluent end, pump and pressurize it into the RAD pressure vessel where it is subject to compressed air pressure. The air pressure then dissolves air into the water to become “saturated recycle”. Once saturated the recycle is introduced into the DAF inlet reaction chamber where it co-mingles with raw incoming water and wastewater. When the recycle is co-mingled with water and wastewater the pressure of the saturated recycle is released and bubbles form and are enmeshed with the wastewater particles.
Recycle Air Dissolving Operation
The RAD creates bubbles by maintaining pressure and an air/water interface in the RAD pressure vessel (stainless steel vertical vessel pictured above right). The interface is maintained by a dual level sensor located in the RAD clear sight tube. When the dual float sensors are wet, the logic says the level is rising and the air pressure supply solenoid is energized and air pressure is added to the RAD vessel. When the dual sensor floats are dry, the logic says the air pressure is excessive and the air supply solenoid is de-energized allowing the level to rise. The level will constantly hunt between (slightly above and below) the two sensor floats. This is normal.
Note: The level sensors MUST be maintained clean. If allowed to foul and malfunction the RAD pressure vessel will fill with water and bubbles will NOT be created. They are easy to remove and clean while the RAD is operating by closing the isolation valves and venting pressure with the sight tube drain valve. The supply air pressure regulator must be maintained at minimum 10 psi above the RAD pressure. If the RAD flow rate valves are adjusted the supply air pressure must be checked to ensure it’
1.2.7 Aeration
Aeration is a unit process in which air and water are brought into intimate contact. Turbulence increases the aeration of flowing streams. Aerators bring water and air in close contact in order to remove dissolved gases (such as carbon dioxide) and oxidizes dissolved metals such as iron, hydrogen sulphide, and volatile organic chemicals (VOCs). Aeration unit is often the first major process at the treatment plant. During aeration, constituents are removed or modified before they can interfere with the treatment processes.
1.2.7.1 Operation and Maintenance of Aerators
Two general methods may be used for the aeration of water. The most common in use is the water-fall aerators. Through the use of spray nozzles, the water is broken up into small droplets or a thin film to enhance counter current air contact. In the air diffusion method of aeration, air is diffused into a receiving vessel containing counter-current flowing water, creating very small air bubbles. This ensures good air-water contact for "scrubbing" of undesirable gases from the water.
1.2.7.2 Operation of Cascade aerator equipment
Cascade Aerators induct air into a water flow in order to oxidize iron and reduce dissolved gases. With Cascade Aerators, aeration is accomplished by natural draft units that mix cascading water with air that is naturally inducted into the water flow. Cascade water is pumped to the top of the aerator, and cascades over a series of trays. Air is naturally inducted into the water flow to accomplish iron oxidation and some reduction in dissolved gasses. Cascade Aerators are of non-corroding, all aluminium or stainless construction and have no moving parts, making them maintenance free and inexpensive to buy and operate.
1.2.7.3 Maintenance of Aeration Equipment
Proper maintenance of aerators is another important area in water treatment activities. Maintenance is on the following elements;
1. Waterfall Aerators
The recommended maintenance procedures for waterfall-type aerators (cascade or step, and tray or splash pan) is as follows:
(a) Weekly
(i) Inspect the aerator surfaces for algae or other growths, precipitated iron oxide, and for non-uniformity of water distribution and staining;
(ii) Clean when necessary;
(iii) Treat with copper sulphate or hypochlorite solution to destroy growths.
(b) Every 6 months
(i) clean and repair tray aerators, removing the trays as necessary;
(ii) Inspect the coke tray aerators for biological growths and coke deterioration;
(iii) Replace the coke if the cleaning is not effective. Repair the screen and enclosures if necessary.
(c) Annually
(i) Repair or replace the surfaces on cascade or step aerators;
(ii) Injection or Diffuser Aerators Injection or diffuser aerators may be either porous medium design or injection nozzles;
(iii) Porous Ceramic Diffusers.
2. Porous ceramic diffusers-plate or tube aerators
The maintenance procedures for porous ceramic diffusers-plate or tube is as follows:
(a) Upon evidence of the non-uniform distribution of air or clogging that impairs operation,
(i) dewater the tank;
(ii) inspect; and
(iii) clean diffusers if necessary.
(b) Every 6 months,
(i) drain the aeration tank and inspect the diffusers for joint leaks, broken diffusers, and clogging;
(ii) Porous ceramic diffusers may suffer clogging of either the waterside or the air side (underside);
(iii) for waterside (porous plate diffusers), use oxidizing acids to clean organic growths from the plate surface.
Note: Chlorine gas introduced into the air line at intervals between inspections will help hold down organic growths. Removable plates should be soaked in 50 percent nitric acid. Plates grouted in place cannot be treated with nitric acid; use chromic acid (made by adding 1 gram of sodium dichromate to 50 ml of sulphuric acid). Pour approximately 2 fluid ounces on each plate 2 days in a row.
Warning: Acids must be handled carefully. DO NOT pour water into sulphuric or chromic acid, as it will explode or splatter. Such acid will cause severe burns to the skin and clothes. ALWAYS pour acid SLOWLY into the water, while stirring continuously. Acid treatment should only be done only under supervision of a chemist or other qualified personnel.
a) Source:http://constructionmanuals.tpub.com/14265/css/Maintenance-of-Aeration-Equipment-294.htm
b) https://cdn2.hubspot.net/hubfs/541513/Brochures/Brochure-Aerators.pdf
1.3 Secondary Treatment
1.3.1 Clarification
Clarification is a process of removing all kind of particles, sediments, oil, natural organic matter and colour from the water to make it clear. A clarification step is the first part of conventional treatment for water and wastewater treatment. It usually consists of physical and/or chemical treatment. Coagulation is normally followed by flocculation in a clarifier, which could be circular or rectangular in shape. After clarification water is then ready for filtration.
1.3.2 Coagulation and Flocculation
The term coagulation and flocculation are often used to describe the process of removal of turbidity caused by fine suspension, colloids and organic colours, i.e. non-settle able particles from water.
1.3.2.1 Coagulation
(a) Chemical Coagulants Commonly Used in Treatment Process
Coagulant chemicals are in two main types including primary coagulants and coagulant aids. Primary coagulants neutralize the electrical charges of particles in the water which causes the particles to clump together well as coagulant aids add density to slow-settling flocs and add toughness to the flocs so that they do not break up during the mixing and settling processes. Primary coagulants are always used in the coagulation/flocculation process while coagulant aids, in contrast, are not always required and are generally used to reduce flocculation time.
Chemically, coagulant chemicals are either metallic salts (such as alum) or polymers. Polymers are man-made organic compounds made up of a long chain of smaller molecules. Polymers can be cationic (positively charged), anionic (negatively charged) or non-ionic (neutrally charged). Table 12.1 shows some of the common coagulant chemicals and lists whether they are used as primary coagulants or as coagulant aids. The various coagulants are used in treatment process. The common coagulants used in water works practices are salt of aluminium viz. filter alum and liquid alum, sodium aluminate, Poly Aluminium Chloride (PAC), Calcium Hydroxide Calcium Oxide and chlorinated copperas which are an equimolecular mixture of ferrous sulphate and ferric chloride being obtained by chlorinating ferrous sulphate.
The commonly used coagulant is commercial grade ferric-alum (solid), However, recently, Poly-Aluminum Chloride is also inducted as a coagulant as it gets properly dispersed, does not have any insoluble residue and effect on the settling tanks, requires less space (<50%). However, it has disadvantage of less effective for colour removal.
Table 12. 1: Types of Coagulants/Aids
- Used as a primary coagulant only in water softening processes.
(Source: Belmont Water Treatment Association as cited in the Water Supply Design Manual, Uganda, 2013).
(b) Tips for Selection of Coagulant
Coagulation is a physical and chemical reactions occurring between the alkalinity of the water and the coagulant added to the water, which results in the formation of insoluble flocs. The most important consideration is the selection of the proper type and amount of coagulant chemical to be added to raw water. Over-dosing as well as under-dosing of coagulants may lead to reduced solids removal efficiency. This condition may be corrected by carefully performed Jar tests and verifying process performance after making any change in the process of the coagulation process.
(c) Aluminium Sulphate Coagulant
Aluminium sulphate is a chemical compound with the formula Al2(SO4)3. Aluminium sulphate is mainly used as a flocculating agent in the purification of drinking water and wastewater treatment plants, and also in paper manufacturing. It is recommended to be used as the coagulant of choice in Tanzania
(d) Use of Aluminium Sulphate
Two solution tanks, one for mixing and the other for dosing, between them holding 48 hours of supply, should be provided. The solution strength should be in the range of 5-10%.The solution tanks could be equipped with hand agitators as shown in Figure 12.2.
Figure 12. 2: Dosing Arrangement for Alum
If the alkalinity of the raw water is low, the pH can be appropriately adjusted by adding soda ash in the correct proportions, as determined after carrying out laboratory experiments called “jar tests”. The strength of the soda ash solution required is usually in the range of 1-10%.The solution tanks for soda ash should also hold a total of 48 hours of supply. The chemical solutions should be fed into the raw water by means of gravity dossers, floating balls or other similar simple devices. Dosing pumps should be used only in exceptional cases.
(e) The Jar Test
To determine the correct chemical dosage for aluminium sulphate solution and for water disinfection, jar testing is recommended (normally Jar test experiments are done in the Laboratory). Jar testing entails adjusting the amount of treatment chemicals and the sequence in which they are added to samples of raw water held in jars or beakers. The sample is then stirred so that the formation, development, and settlement of floc can be watched just as it would be in the full scale treatment plant. Jar testing should be done seasonally (temperature), monthly, weekly, daily, or whenever a chemical is being changed, or new pumps, rapid mix motors, new floc motors, or new chemical feeders are installed. There is no set requirement for how often jar testing should be conducted, but the more it is done the better the plant will operate. Optimization is the key to running he plant more efficiently.
(f) Dosing of the coagulant at a spot of maximum turbulence Rapid mix of coagulant at a spot of maximum turbulence, followed by tapered flocculation in three compartmentalized units allows a maximum of mixing(reduced short circuiting), followed by a period of agglomeration intended to build larger fast settling flocs.
(g) Mixing The mixing is the process to mix all the coagulant in water rapidly and instantaneously especially in waters with high alkalinity so as to achieve complete homogenization of a coagulant in the water to be treated. Mixing of the coagulant can be satisfactorily accomplished in a special coagulant tank with mixing devices or in the influent channel or a pipeline to the flocculation basin with high flow velocity which produces necessary turbulence.
To accomplish the mixing, following methods can be used:
(i) Hydraulic mixing,
(ii) Mechanical mixing,
(iii) Diffusers and grid system,
(iv) Pump-blenders.
(h) Storage of Aluminium Sulphate
Aluminium sulphate should be stored in a secured, cool, dry, well-ventilated area, removed from oxidising agents, alkalis, most metals, heat or ignition sources and foodstuffs. Ensure containers are adequately labelled, protected from physical damage and sealed when not in use. Check regularly for leaks or spills (if in a solution form). Large storage areas should have appropriate fire protection and ventilation systems.
(i) Health Hazards and Disposal of Waste Solution and Sludge
Aluminium sulphate is categorised as a slightly corrosive, irritant and hazardous substance. This product has the potential to cause adverse health effects with over long exposure time. Use safe work practices to avoid eye or skin contact and inhalation. It may hydrolyse (with addition of water) to sulphuric acid, a strong tissue irritant. If released to water environment; aluminium salts will slowly be precipitated as aluminium hydroxide. This may lower the pH of receiving waters with toxic effects to aquatic organisms. It is not expected to bio- accumulate. Water plants may experience chronic toxicity at around 25 ppm. Before disposal, neutralise the solution with lime, weak alkali or similar. For small amounts, absorb with sand or similar and dispose of to an approved landfill site
1.3.2.2 Flocculation
(a) Flocculation Basin– Operation
The objective of a flocculation basin is to produce a settled water of low turbidity which in turn leads to reasonably longer service period of filter plant.
(b) Clari-flocculator
The flocculators may be circular, square or rectangular. The best flocculation is usually achieved in a compartmentalized basin. The compartments (most often three) are separated by baffles to prevent short circuiting of the water being treated. The turbulence can be reduced gradually by reducing the speed of the mixers in each succeeding tank or by reducing the Surface area of the paddles. This is called tapered-energy mixing. The reason for reducing the speed of the stirrers is to prevent breaking apart the larger flocs, which have already formed. If the floc is broken up nothing is accomplished and the filter gets overloaded.
(c) Coagulation – Flocculation Process Action
Typical jobs performed by an operator in the normal operation of the coagulation-flocculation process include the following:
(i) Monitor process performance,
(ii) Evaluate water quality conditions (raw and treated water),
(iii) Check and adjust process controls and equipment, and
(iv) Visually inspect facilities.
(d) Interaction with Sedimentation and Filtration
The processes of coagulation-flocculation are required to precondition or prepare non settle able particles present in the raw water for removal by sedimentation and filtration. Small particles (particularly colloids), without proper coagulation-flocculation are too light to settle out and will not be large enough to be trapped during filtration process. Since the purpose of coagulation–flocculation is to accelerate particle removal, the effectiveness of the sedimentation and filtration processes, as well as overall performance depends upon successful coagulation - flocculation.
(e) Examination of the Floc
• Examine the water samples at several points, en-route the flow line of the water. Look at the clarity of the water between the flocs and study the shape and size of the flocs. Observe the floc as it enters the flocculation basins which should be small and well dispersed throughout the flow;
• Tiny alum floc may be an indication that the chemical dose is too low. A ‘popcorn flake’ is a desirable floc. If the water has a milky appearance or a bluish tint, the alum dose is probably too high. As the floc moves through the flocculation basins, the size of the floc should be increasing. If the size of the floc increases and then later starts to break up, the mixing intensity of the downstream flocculator may be too high. Thus, the speed of these flocculators needs to be reduced or otherwise the coagulant dosage may be increased;
• Examine the settlement of the floc in the sedimentation basin. If a lot of flocs are observed flowing over the laundering weirs the floc is too light for the detention time. By increasing the chemical dose or adding a coagulant aid such as a polymer to produce heavier and larger flocs. The appearance of the fine floc particles passing over the weir could be an indication of too much alum and the dose should be reduced. For precise evaluation only one change can be made at a time and evaluate the results.
(f) Record keeping
Records of the following items should be maintained:
• Source water quality (pH, turbidity, temperature, alkalinity, chlorine demand and colour;
• Process water quality (pH, turbidity, and alkalinity);
• Process production inventories (chemicals used, chemical feed rates, amount of water processed, and amount of chemicals in storage);
• Process equipment performance (types of equipment in operation, maintenance procedures performed, equipment calibration and adjustments);
• A plot of key process variables should be maintained. A plot of source water turbidity vs. coagulant dosage should be maintained. If other process variables such as alkalinity or pH vary significantly, these should also be plotted.
(g) Safety considerations
In the coagulation-flocculation processes, the operator may be exposed to the associated hazards with following:
• Electrical equipment,
• Rotating mechanical equipment,
• Water treatment chemicals,
• Laboratory reagents (chemicals),
• Slippery surfaces caused by certain chemicals,
• Flooding,
• Confined spaces and underground structures such as valve or pump vaults (toxic and explosives gases, insufficient oxygen).
Strict and constant attention must be given to safety procedures. The operator must be trained with general first aid practices such as mouth-to-mouth resuscitation, treatment of common physical injuries, and first aid for chemical exposure (chlorine).
(h) Laboratory Tests
Water quality indicators for the operation of flocculation process include turbidity, alkalinity, chlorine demand, residual chlorine test, colour, pH, temperature, odour and appearance and need to be tested. In multi-habitation or big schemes, a provision of automatic water testing equipment or onsite laboratory at treatment plant may be established and maintained for the purpose.
1.3.2.3 Rapid Sand Filtration Plant
This is a process in which water flows onto the top of the filter media and is driven through it by gravity. In passing through the small spaces between the filter's sand grains, impurities are removed. The water continues its way through the support gravel, enters the under-drain system, and then flows to the reservoir. It is the filter media which actually removes the particles from the water. The filter media is routinely cleaned by means of a backwashing process.
Rapid sand filtration (RSF) is a relatively sophisticated process usually requiring power-operated pumps for backwashing or cleaning the filter bed, and some designs require flow control of the filter outlet. A continuously operating filter will usually require backwashing about every two days or so when raw water is of relatively low turbidity and at least daily during periods of high turbidity. Because of the higher filtration rates, the area requirement for a rapid gravity filtration plant is about 20% of that required for Slow Sand Filters (SSF).
Initial filtering performance can be re-achieved through a cleaning of the filter bed. This is usually conducted through backwashing: the flow of water is reversed, so that treated water flows backwards through the filter. The sand is re-suspended and the solid matter is separated in the surface water. Often, air is injected additionally to support the cleaning process (WHO 1996). As soon as most particles are washed out and the backward flowing water is clear, the filter is put back to operation. Clearly, relatively large quantities of sludge are generated through backwashing and require some form of treatment before discharge into the environment (UNEP 1998).
Rapid sand filtration is a highly effective method to remove turbidity if it is correctly applied (Brikke & Bredero 2003). Equally, solids formed during pre-treatment, i.e. coagulation-flocculation, are filtered. A well-operated RSF reduces turbidity to less than 1 NTN and often less than 0.1 NTU (WHO 1996). Regarding the removal of most other contaminants, the RSFs are ineffective. If combined with adequate pre-treatment measures and final disinfection, rapid sand filtration usually produces safe drinking water.
(a) Filter sand
Filter sand is defined in terms of effective size and uniformity coefficient. Effective size is the sieve size in mm that permits 10% by weight to pass. Uniformity in size is specified by the uniformity coefficient which is the ratio between the sieve sizes that will pass 60% by weight and the effective size.
Check shape size and quantity of filter sand to the followings:
(i) Sand shall be of hard and resistant quartz or quartzite and free of clay, fine particles, soft grains and dirt of every description,
(ii) Effective size shall be 0.4 to 0.7 mm,
(iii) Uniformity coefficient shall not be more than 1.7 nor less than 1.3,
(iv) Ignition loss should not exceed 0.7 per cent by weight,
(v) Soluble fraction in hydrochloric acid shall not exceed 5.0% by weight,
(vi) Silica content should be not less than 90%,
(vii) Specific gravity shall be in the range between 2.55 to 2.65,
(viii) Wearing loss shall not exceed 3%.
1.3.2.3.1 Interaction with Other Treatment Processes
The purpose of RSF is to remove particulate impurities and floc from the raw water. In this regard, the filtration process is the final step in the solids removal process which usually includes the pre-treatment processes of coagulation, flocculation and sedimentation. The degree of treatment applied prior to filtration depends on the quality of water.
1.3.2.3.2 Operation and Backwashing
Rapid Sand Filters should be washed before placing them into service. (a) A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process should be stopped and the filter be taken out of service for cleaning or backwashing;
(b) The surface wash system should be activated just before the backwash cycle starts to aid in removing and breaking up solids on the filter media and to prevent the development of mud balls. The surface wash system should be stopped before completion of the back-wash cycle to permit proper settling of the filter media;
(c) A filter wash should begin slowly for about one minute to permit removing of an entrapped air from the filter media, and also to provide uniform expansion of the filter bed. After this period, the full backwash rate can be applied. Sufficient time should be allowed for cleaning of the filter media. Usually when the backwash water coming up through the filter becomes clear, the media is washed. This generally takes from 3 to 8 minutes. If flooding of wash water troughs or carryover of filter media is a problem, the backwash rate must be reduced.
A filter is usually operated until just before clogging or breakthrough occurs or a specified time period has passed (generally 24 hours). After a filter clogs/breakthrough occurs, the filtration process is stopped and the filter is taken out of service for cleaning or backwashing.
Surface Wash: In order to produce optimum cleaning of the filter media during backwashing and to prevent mud balls, surface wash (supplemental scouring) is usually practiced. Surface wash systems provide additional scrubbing action to remove attached floc and other suspended solids from the filter media.
1.3.2.3.3 Operation and Maintenance of Rapid Sand Filters
Operation of a rapid sand filter consists of flow control, regular backwashing and cleaning. The period between backwashes depends on the quality of the influent water and normally lies between 24 – 72 hours (UNEP 1998).The cleaning process requires an interruption of the purification process of 5 - 10 minutes per filter bed. Several parallel filter units are required to guarantee constant water supply. The backwash process must be observed carefully; in particular the rate of flow must be controlled to avoid erosion of the filter medium. Periodic repacking of the filter bed may be required at infrequent intervals to ensure efficient operation (UNEP 1998). Operation and maintenance thus requires skilled and highly reliable workers. Table 12.2 illustrate the details of RSF operation and maintenance.
Table 12. 2: Operation and Maintenance Details for RSF
1.3.2.3.3.1 Operating Procedures
From a water quality point of view, filter effluent turbidity is a good indication of overall process performance. However, monitoring the performance of each of the individual water treatment process including sedimentation is must in order to check water quality or performance changes. Operations are considered to be normal within the operating ranges of the plant, while unusual or difficult to handle condition is abnormal operating condition. In normal operation of the sedimentation process one must monitor the following;
(a) Turbidity of inflow and out flow of Water in the Sedimentation Basin: Turbidity of inflow water indicates the floc or solids loading to the sedimentation basin while turbidity of outflow water of the basin indicates the effectiveness or efficiency of the sedimentation process. Low levels of outflow water turbidity to be achieved to minimize the floc loading on the filter.
(b) Temperature of inflow water: is important as the water becomes colder, the settling of particles become slow. To compensate for this change, jar tests should be performed and accordingly, the coagulant dosage is to be adjusted to produce a heavier and thus a settle-able floc. Another possibility is to provide longer detention times when water demand decreases.
(c) Visual checks of the sedimentation process: should include observation of floc settling characteristics, distribution of floc at the basin inlet and clarity of outflow settled water spilling over the weirs. An uneven distribution of floc or poorly settling floc is an indication of a raw water quality change or there is operational problem.
(d) Process Actions/ steps are as indicated below:
(i) Monitor process performance.
(ii) Evaluate turbidity and make appropriate process changes.
(iii) Check and adjust processes equipment (change chemical feed rates).
(iv) Backwash filters.
(v) Evaluate filter media condition (media loss, mud balls, cracking),
(vi) Visually inspect facilities.
1.3.2.3.3.2 Important Process Activities and Precautions
Process performance monitoring is an on-going activity. Check for any treatment process changes or other problems which might affect filtered water quality, such as a chemical feed system failure. Measurement of head-loss built up in the filter media may give a good indication of how well the solids removal process is performing. The total designed head loss from the filter influent to the effluent in a gravity filter is usually about 3 meters. At the beginning of the filtration cycle the actual measured head loss due to clean media and other hydraulic losses are about 0.9 m. This would permit an additional head-loss of about 2.1 m due to solid accumulation in the filter.
The rate of head-loss build up is an important indication of process performance. Sudden increase in head loss might be an indication of surface sealing of the filter media (lack of depth penetration).Early detection of this condition may require appropriate process changes such as adjustment of chemical filter aid feed rate or adjustment of filtration rate. Monitoring of filter turbidity on a continuous basis with an online turbidity meter may be adopted for obtaining continuous feedback on the performance of the filtration process. In most instances it is desirable to cut off (terminate) filter at a predetermined effluent turbidity level. Preset the filter cut-off control at a point where breakthrough occurrence is noticed/ tested.
In the filter process, time for completion of normal filter process may be calculated on the basis of the following parameters:
(a) Head-loss;
(b) Effluent turbidity level;
(c) Elapsed run time;
(d) A predetermined value established for each above parameter as a cut off point for filter operation may be checked and when any of the selves is reached, the filter should be removed from service and backwashed;
(e) At least once a year, the filter media must be examined and evaluate its overall condition;
(f) Measure the filter media thickness for an indication of media loss during the back-washing process;
(g) Mud ball accumulation in the filter media to evaluate the effectiveness of the overall back-washing operation.
1.3.2.3.3.3 Routine observations
(a) The backwash process to qualitatively assess process performance,
(b) For media boils (uneven flow distribution) during backwashing, media carry over in to the wash water trough, and
(c) Clarity of the waste wash-water near the end of the backwash cycle,
(d) Upon completion of the backwash cycle, observe the condition of the media surface,
(e) Check for filter sidewall or media surface cracks,
(f) Routinely inspect physical facilities, equipment as part of good house-keeping and maintenance practices,
(g) Correct or report the abnormal equipment conditions to the water supply utility/agency for maintenance action.
Never bump upon filter to avoid back-washing. Bumping is the act of opening the backwash valve during the course of a filter run to dislodge the trapped solids and increase the length of filter run. This is not a good practice. Shortened filter runs can occur because of air bound filters. Air binding will occur more frequently when large head losses are allowed to develop in the filter. Precautions should be taken to minimize air binding to avoid damage to the filter media.
1.3.2.3.3.4 Record Keeping
A daily operations log of process performance data and water quality characteristics shall be recorded and maintained accurately for the following items:
(a) Process water quality (turbidity, colour, PH and alkalinity);
(b) Process operation (filters in service, filtration rates, loss of head, length of filter runs, frequency of backwash, backwash rates, and UFRV unit filter run volume);
(c) Process water production (water processed, amount of backwash water used, and chemicals used);
(d) Percentage of water production used to backwash filters;
(e) Process equipment performance (types of equipment in operation, equipment adjustments, maintenance procedures performed, and equipment calibration).
1.3.2.3.3.5 Start-up and Shutdown Procedures
(a) Routine Procedures Most plants keep all filters into service except unit under backwash operation and maintenance. Filter units are routinely taken off line for backwashing when the media becomes clogged with particulates, turbidity break through occurs or demands for water are reduced.
(b) Implementation of Start-up and Shut-down Procedures
Filter check-out procedures:
(i) Check operational status of filter;
(ii) Be sure that the filter media and wash water troughs are clean of all debris such as leaves, twigs, and tools;
(iii) Check and be sure that all access covers and walk-way gratings are in place;
(iv) Make sure that the process monitoring equipment such as head-loss and turbidity systems are operational;
(v) Check the source of back-wash to ensure that it is ready to go.
1.3.2.3.3.6 Preventive Maintenance Procedures
Preventive maintenance programmes are to assure the continued satisfactory operation of treatment plant facilities by reducing the frequency of break-down failures. Routine maintenance functions of operator may include:
(i) Keeping electric motors free of dirt, moisture and pests (rodent sand birds);
(ii) Assuming good ventilation (air circulation) in equipment work areas;
(iii) Checking pumps and motors for leaks, unusual noise and vibrations or overheating;
(iv) Maintaining proper lubrication and oil levels;
(v) Inspecting for alignment of shafts and couplings;
(vi) Checking bearings for overheating and proper lubrication;
(vii) Checking the proper valve operation (leakage or jamming);
(viii) Checking automatic control systems for proper operation;
(ix) Checking air/vacuum relief systems for proper functioning, dirt and moisture;
(x) Verifying correct operation of filters and back-washing cycles by observation;
(xi) Inspecting filter media conditions (look for algae and mud balls and examine gravel and media for proper gradation);
(xii) Inspecting filter underdrain system (be sure that the under drain openings are not becoming clogged due to media, corrosion nor chemical deposits).
1.3.2.3.3.7 Safety Considerations
(a) Electrical Equipment
(i) Avoid electric shock (use preventive gloves),
(ii) Avoid grounding yourself in water or on pipes,
(iii) Ground all electric tools,
(iv) Lock-out and tag electrical switches and panels when servicing equipment.
(b) Mechanical Equipment
(i) Use protective guards on rotating equipment,
(ii) Don’t wear loose clothing around rotating equipment,
(iii) Keep hands out of energized valves, pumps and other pieces of equipment,
(iv) Clean –up all lubricant and chemicals pills (slippery surfaces cause bad falls).
(c) Open – Surface Filter
(i) Use safety devices such as hand rails and ladders,
(ii) Close all openings and replace safety gratings when finished working,
(iii) Know the location of all life preservers and other safety devices.
(d) Valve and Pump Vaults, Sumps, Filter galleries
(i) Be sure that all underground or confined structures are free of hazardous atmospheres (toxic or explosive gases, lack of oxygen)by checking with gas detectors,
(ii) Work in well ventilated structures (use air circulation fans).
1.3.2.4 Sedimentation
Sedimentation tank, also called settling tank or clarifier, component of a modern system of water supply or wastewater treatment. A sedimentation tank allows suspended particles to settle out of water or wastewater as it flows slowly through the tank, thereby providing some degree of purification. The purpose of sedimentation process is to remove suspended particles so as to reduce load on Filters. If adequate detention time and basin surface area are provided in the sedimentation basins, solids removal efficiencies can be achieved more than 95%. However, it may not always be the cost effective way to remove suspended solids.
In low turbid water sources (less than about 10 NTU) effective coagulation, flocculation and filtration may produce satisfactory filtered water without sedimentation. In this case, coagulation-flocculation process is operated to produce a highly filterable tiny floc, which does not readily settle due to its small size; instead the tiny floc is removed by the filters. There is, however, a practical limitation in applying this concept to higher turbidity conditions. If the filters become overloaded with suspended solids, they will quickly clog and need frequent back washing. This can limit plant production and cause degradation in filtered water quality. Thus, the sedimentation process should be operated from the standpoint of overall plant efficiency. If the source water turbidity is only 3 mg/l, and the jar tests indicate that 0.5 mg/l of coagulant is the most effective dosage, then one cannot expect the sedimentation process to remove a significant fraction of the suspended solids. On the other hand, source water turbidity in excess of 50 mg/l will probably require a high coagulant dosage for efficient solids removal and the suspended particles and alum floc should be removed by sedimentation basin.
1.3.2.4.1 Sedimentation Basins
The Basin can be divided into four zones viz. Inlet; Settling; Sludge and Outlet zone. The basins may be of the following types:
(a) Rectangular basins,
(b) Circular and square basins,
(c) High Rate Settlers (Tube Settlers),
(d) Solid Contact Units (Up-flow solid-contact clarification and up-flow sludge blanket clarification).
1.3.2.4.2 Process Actions
In rectangular and circular sedimentation basins, it is generally possible to make a judgment about the performance of the sedimentation process by observing how far the flocs are visible beyond the basin inlet. When sedimentation is working well, the floc will only be visible for short distance. When the sedimentation is poor, the floc will be visible for a long distance beyond the inlet.
In up-flow or solid-contact clarifiers, the depth of the sludge blanket and the density of the blanket are useful monitoring tools. If the sludge blanket is of normal density (measured as milligrams of solids per litre of water) but is very close to the surface, more sludge should be wasted. If the blanket is of unusually light density, the coagulation-flocculation process (chemical dosage) must be adjusted to improve performance.
With any of the sedimentation processes, it is useful to observe the quality of the effluent as it passes over the weir. Flocs coming over at the ends of the basin are indicative of density currents, short circuiting, sludge blankets that are too deep or high flows. The clarity of the outflow is also a reliable indicator of coagulation-flocculation efficiency. Process equipment should be checked regularly to assure adequate performance. Proper operation of sludge removal equipment should be verified each time for its operation, since sludge removal piping systems are subject to clogging. Free flowing sludge can be readily observed if sight glasses are incorporated in the sludge discharge piping. Otherwise, the outlet of the sludge line should be observed during sludge pumping. Frequent clogging of sludge pipe requires increasing frequency of sludge removal equipment and this can be diagnosed by performing sludge solids volume analysis in the laboratory.
1.3.2.4.3 Sludge Management
1.3.2.4.3.1 Sludge characteristics
Water treatment sludge is typically alum sludge, with solid concentrations varying from 0.25 to 10% when removed from a basin. In gravity flow sludge removal systems, the solid concentration should be limited to about 3%. If the sludge is to be pumped, solids concentrations should be high as 10% for readily transportation. In horizontal flow sedimentation basins preceded by coagulation and flocculation, over 50% of the floc will settle out in the first third of the basin length. Operationally, this must be considered when establishing the frequency of the operation of sludge removal equipment.
1.3.2.4.3.2 Sludge Removal Systems
Sludge which accumulates on the bottom of the sedimentation basins must be removed periodically for the following reasons:
• To prevent interference with the settling process (such as re-suspension of solids due to scouring);
• To prevent the sludge from becoming septic or providing an environment for the growth of microorganisms that create taste and odour problems;
• To prevent excessive reduction in the cross sectional area of the basin (reduction of detention time).
In large scale plants, sludge is normally removed on an intermittent basis with the aid of mechanical sludge removal equipment. However, in smaller plants with low solid loading, manual sludge removal may be more cost effective. In manually cleaned basins, the sludge is allowed to accumulate until it reduces settled water quality. High levels of sludge reduce the detention time and floc carries over to the filters. The basin is then dewatered (drained), most of the sludge is removed by stationary or portable pumps, and the remaining sludge is removed with squeegees and hoses. Basin floors are usually sloped towards a drain to help sludge removal. The frequency of shutdown for cleaning will vary from several months to a year or more, depending on source water quality (amount of suspended matter in the water).
In larger plants, a variety of mechanical devices can be used to remove sludge including:
• Mechanical rakes,
• Drag-chain and flights,
• Travelling bridge.
Circular or square basins are usually equipped with rotating sludge rakes. Basin floors are sloped towards the centre and the sludge rakes progressively push the sludge toward a centre outlet. In rectangular basins, the simplest sludge removal mechanism is the chain and flight system.
1.3.2.4.3.3 Sludge Disposal
Disposal of waste from the water treatment plants has become increasingly important with the availability of technology and the need for protection of the environment. Treatment of waste solid adds to the cost of construction and operation of treatment plants.
Waste from the Water treatment plants comprise of:
• Sludge from sedimentation of particulate matter in raw water, flocculated and precipitated material resulting from chemical coagulation, or residuals of excess chemical dosages, plankton, etc.,
• Waste from rinsing backwashing of filter media containing debris, chemical precipitates, straining of organic debris and plankton and residual of excess chemical dosages, etc.; and
• Waster from regeneration processes of ion exchange softening treatment plant containing cation of calcium, magnesium and unused sodium and anion of chlorides and sulphates originally present in the regenerate.
1.3.2.4.3.4 Disposal Method
In continuous sludge removal, the feasibility of discharging of water treatment plant sludge to existing sewer nearby should be considered. For lime softening plant sludge, the reclamation by calcining and reuse can be explored. These sludge from clarification units using irons and aluminium coagulant can be dewatered by vacuum filtration. However the method of waste disposal shall conform to the pollution control norms.
1.3.2.4.3.5 Reuse of Sludge
A large quantity of sludge is generated each year from water treatment plants in Tanzania. Disposing the sludge to the nearest watercourse is the common practice, especially by many urban water utilities, which accumulatively rise the aluminum concentrations in water and consequently in human bodies. Landfill disposal of the sludge is impractical because of the high cost of transportation and depletes the capacity of the landfill. The use of sludge in construction industry is considered to be the most economic and environmentally sound option. Due to the similar mineralogical composition of clay and water treatment plant sludge, various researchers have studied on the reuse of sludge in clay-brick production as a partial substitute for clay in brick manufacturing. However, concluded that by operating at the temperature commonly practiced in the brick kiln, 50 percent was the optimum sludge addition to produce brick from sludge-clay mixture. The produced bricks properties have proved superior to those available in the market. (Source: https://www.researchgate.net/publication/295548404_Reuse_of_Water_Treatment_Plant_Sludge_in_Brick_Manufacturing)
1.3.2.4.4 Start-up and Shutdown Procedures
In the event of requirement for shut down or start-up of processes on account of maintenance or a major equipment failure, proper procedures must be followed as per recommendations of the manufacturer of the plant and equipment. The procedures, in general, are given below:
(a) Start up Procedure
(i) Check operational status, mode of operation of equipment and physical facilities:
• Check that basin valves are closed,
• Check that basin isolation gates are closed,
• Check that launder weir plates are set at equal elevations,
• Check to ensure that all trash, debris and tools have been removed from basin.
(ii) Test sludge removal equipment:
• Check that mechanical equipment is properly lubricated and ready for operation,
• Observe operation of sludge removal equipment.
(iii) Sedimentation basin filled with water:
• Observe proper depth of water in basin,
• Remove floating debris from basin water surface.
(iv) Start sample pumps,
(v) 5) Perform water quality analyses,
(vi) Operate sludge removal equipment. Be sure that all valves are in the proper position & operational.
(b) Shut down Procedures
(i) Stop flow to sedimentation basin. Install basin isolation gates,
(ii) Turn off sample pump,
(iii) Turn off sludge removal equipment,
(iv) Shut off mechanical equipment and disconnect where appropriate,
(v) Check that valves are in proper position& operational,
(vi) Lock out electrical switches and equipment,
(vii) Dewater basin, if necessary;
(viii) Be sure that the water table is not high enough to float the empty basin.
(ix) Open basin drain valves,
(x) Grease and lubricate all gears, sprockets and mechanical moving parts which have been submerged immediately following dewatering to avoid seize up.
1.3.2.4.5 Equipment
(a) Types of support equipment – Operation and Maintenance
The operator should be thoroughly familiar with the operation and maintenance instructions issued by the manufacturer for each specific equipment viz. flow meters and gauges valves control systems; water quality monitors such as turbidity meters; sludge removal equipment; sludge and sump pumps.
Equipment Operation
(i) Check the following: Proper lubrication and operational status of each unit,
(ii) Excessive noise and vibration, overheating and leakage,
(iii) Pumps suction and discharge pressure.
1.3.2.4.6 Safety Considerations
(a) Electrical Equipment
(i) Avoid electric shock,
(ii) Avoid grounding yourself in water or on pipes,
(iii) Ground all electric tools,
(iv) Use a lock out and tag system for electric equipment or electrically driven mechanical equipment.
(b) (ii) Mechanical Equipment
(i) Keep protective guards on rotating equipment,
(ii) Do not wear loose clothing around rotating equipment,
(iii) Keep hands out of valves, pumps and other equipment,
(iv) Clean up all lubricant and sludge spills.
(c) (iii) Open Surface water – filled structures
(i) Use safety devices such as hand rails and ladders,
(ii) Close all openings,
(iii) Know the location of all life preservers.
(d) Valve and Pump Vaults, Sumps
(i) Be sure all underground or confined structures are free of hazardous atmosphere (Toxic or explosive gases, lack of oxygen),
(ii) Work only in well ventilated structures,
(iii) Take proper steps against flooding.
1.3.2.4.7 Corrosion Control
All metallic parts which are prone to corrosion must be protected. Corrosion can be controlled to a large extent by applying anti corrosive paints on the steel pipes at the time of construction of the borehole. Non-corrosive casing pipe and strainers (Such as PVC pipes and strainers) can also be used at the time of construction of borehole to avoid corrosion. Some commonly used paints/coatings to control corrosion are of aluminium, asphalt, red lead and coal tar.
1.3.2.4.8 Preventive Maintenance
Such programmes are designed to assure the continued satisfactory operation of treatment plant by reducing the frequency of breakdown failures. Typical steps should include:
(a) Keeping electric motors free of dirt and moisture;
(b) Assuring good ventilation at valve and pump vaults, sumps;
(c) Checking pumps and motors for leaks, unusual noise and vibrations, overheating or signs of wear;
(d) Maintaining proper lubrication and oil levels;
(e) Inspecting alignment of shafts and couplings;
(f) Checking bearings for overheating and proper lubrication;
(g) Checking for proper valve operation;
(h) Checking for free flow of sludge in sludge removal collection and discharge systems;
(i) Good housekeeping.
1.3.2.5 Operation and Maintenance for Defluoridation
Fluoride compounds, usually calcium fluoride, are naturally found, usually in low concentration in water. However, water from underground sources can have higher levels of fluoride to the level that it becomes a health hazard. Defluoridation process is both difficult and expensive, more details and standards can be found in Volume I.
The decision on whether or not to include defluoridation in a water supply scheme considers both number of potential consumers, alternative sources, the financial consequences both in capital and running and whether or not there is a possibility to dilute the water containing the fluoride as a means of reducing the concentration. Defluoridation is necessary when the fluoride concentration is higher than acceptable limits. The methods presented in Volume I may be considered for attaining defluoridised water.
For the absorption method, the following are the procedures:
(a) Check filter against growth of algae (if exposed to sunlight),
(b) Check blocked filter by sedimentation,
(c) Check fluoride saturation,
(d) Check treated water quality (fluoride concentration) once per quarter.
1.3.3 Tertiary Treatment
1.3.3.1 Disinfection
Drinking water is disinfected to kill bacteria, viruses and parasites, which may exist in the water and may cause illness and disease like Campylobacter, Cholera, Amoebic Dysentery, Giardia (beaver fever) and Cryptosporidium. These organisms usually get into drinking water supplies when source of waters such as lakes or streams, community water transmission pipes or storage reservoirs are contaminated by animal waste or human sewage. Generally, deep wells are safer than shallow wells if chemical contamination is absent. In fact, shallow dug wells are often as contaminated as lakes or streams. The disinfection of potable water is almost universally accomplished by the use of gaseous chlorine or chlorine compounds. Chlorine is easy to apply, measure and control. It persists reasonably well and it is relatively inexpensive. Other methods of disinfection are also available viz. ozone, ultra-violet light, chlorine dioxide, silver ionization.
1.3.3.2 Chlorination
The primary objectives of the chlorination process are disinfection, taste and odour control in the system, preventing the growth of algae and other micro-organisms that might interfere with coagulation and flocculation, keeping filter media free of slime growths and mud balls and preventing possible built up of anaerobic bacteria in the filter media, destroying hydrogen sulphide and controlling sulphurous taste and odour in the finished water, removing iron and manganese, bleaching of organic colour.
Dosage: Effective chlorine dose should be such that sufficient chlorine is there to react with organic matter, ammonia, iron, manganese and other reducing substances in water and at the same time leave sufficient chlorine to act as algaecide. Dose required for this purpose may be over 2mg/L Post chlorination dose can be adjusted to obtain minimum 0.2to 0.5 mg/l residual chlorine in potable water at consumer end.
1.3.3.2.1 Effectiveness of Chlorination
Generally, chlorination without filtration or other pre-treatment is effective and adequate only under the following conditions:
(a) The degree of bacteriological pollution of the water is moderate, reasonably uniform, and not imbedded in suspended solids, for example, within the bodies of worms;
(b) The turbidity and colour of the water do not exceed 5-10 units;
(c) The content of iron or manganese or both do not exceed 0.3 mg/L; and
(d) Taste- or odour-producing substances are absent or do not require chlorine doses that inevitably produce a chlorine taste in the treated water.
There is a contact period of at least 20 minutes between the point of chlorination and the first service connection supplied with the water. In cases where water is pumped directly from the source (e.g. well) into the distribution system, chlorine may be applied.
1.3.3.2.2 Chlorination Guideline
(a) Chlorine solutions lose strength while standing or when exposed to air or sunlight. Make fresh solutions frequently to maintain the necessary residual.
(b) Maintain a free chlorine residual of 0.3 mg/l after 30 minutes contact time. Residual chlorine should be measured every day.
(c) Once the chlorine dosage is increased to meet greater demand, do not decrease it unless the raw water quality improves.
(d) When there is a risk of cholera or an outbreak has already occurred, maintain the chlorine residuals as follows:
(i) Distribution system: 0.5 mg/l
(ii) Tanker trucks at filling point: 2 mg/l
1.3.3.2.3 Chlorination Methods
Disinfection is carried out by applying chlorine or chlorine compounds. The methods of application are as follows:
(a) Preparing weak solution by bleaching powder,
(b) Preparing weak solution by electrolysing brine solution,
(c) By adding chlorine either in the form of gas or solution prepared from dissolving chlorine gas in small feed of water.
1.3.3.3 Disinfection by Bleaching Powder
Bleaching powder or calcium hypochlorite is a chlorinated lime, which contains about 25 to 34% of available chlorine by weight. Chlorine being a gas is unstable and as such it is mixed with lime to retain its strength for a longer period, as far as possible. The bleaching powder is hygroscopic in nature. It loses its chlorine strength rapidly due to poor storage and hence should not be stored for more than three months. The method of chlorination by bleaching powder is known as hypo-chlorination. The combined action of hypochlorous acid and hypochlorite ion brings about the disinfection of water.
1.3.3.3.1 Preparation of Solution
The concentrated solution of bleaching powder is prepared in one or two tanks of capacity suitable for 24 hours requirement. The tank inside should be of glazed tiles or stoneware and should be covered. The tank should be under shade and not direct to sunlight.
(a) The powder is first put on a perforated slab placed longitudinally inside the tank at a higher level, with respect to bed level of tank;
(b) Water is sprinkled on the powder through a perforated pipe above this perforated slab;
(c) The solution of bleaching powder and water now enters the tank. The solution is rotated for thorough mixing of powder with water by a hand driven/motor reduction gear operated slow speed stirrer is now ready for use as disinfectant;
(d) The precipitates of calcium hydroxide settle at the bottom of the tank. The supernatant water, which contains OCl, Cl- plays important role in disinfection;
(e) For effectiveness of chlorination, contact period of at least 4 hours should be maintained.
1.3.3.3.2 Dosing of Solution
The solution is discharged to a small measuring tank at a lower level through PVC pipe or any other material resistant to chlorine. The level of water in this tank is maintained constant through a float valve. The solution is dosed to the clear water channel by gravity at the time of entry to clear water reservoir. The dose has to be monitored properly, depending on the desired residual chlorine required in clear water reservoir. The waste precipitates at the bottom of tanks are taken out occasionally by scour valve.
1.3.3.3.3 Safety precautions
(a) The operating personnel should use hand gloves, aprons and other protective apparel, while handling and mixing;
(b) The valves, stirrer, tanks, plumbing arrangements require renovation at every 6 months or so.
1.3.3.4 Chlorination by Gaseous Chlorine
Elemental chlorine at a normal pressure is a toxic, yellow green gas, and is liquid at high pressure. Chlorine gas is released from a liquid chlorine cylinder by a pressure reducing and flow control valve operating at a pressure less than atmospheric pressure. The gas is injected in the water supply pipe where highly pressurized water is passed through a venture creating a vacuum that draws the chlorine in to the water stream. Adequate mixing and contact time must be provided after injection to ensure complete disinfection of pathogens. It may be necessary to control the water pH. A basic system consists of chlorine cylinder mounted with vacuum regulator, chlorine gas injectors, and a contact tank or pipe. Prudence or state regulation would require that a second cylinder and gas regulator be provided with a changeover valve to ensure continuity of disinfection. Additional safety and control system may be required.
Chlorine is very effective for removing almost all pathogen and is appropriate for both a primary and secondary disinfectant. The limitation with this is it is dangerous gas that is lethal at concentrations as low as 0.1 per cent air by volume.
1.3.3.4.1 Working Safely around Chlorine Gas
Any water utility that uses chlorine should have written procedures for its chlorine system operation. Even the use of powdered chlorine should have written procedures. Before starting any chlorination process, the following precautions should be taken:
(a) If a faucet with good flowing water is not available close by, make ready a 5- gallon container of fresh water, but make sure it is away from the chlorine cylinder or storage area. This is to ensure that if the chlorine accidentally comes in contact with your eyes or skin, you can flush the affected areas with copious amounts of fresh water for at least 10-15 minutes,
(b) Flush the chlorine out. Do not just soak the affected surface. If you get some of the chlorine solution in your eyes, flush it out and immediately see your doctor,
(c) Wear the prescribed safety clothing and equipment, specifically:
(i) Goggles to protect your eyes from contact with the chlorine in any form,
(ii) Rubber gloves and rubber boots certified for use around the chemical to protect your hands and feet,
(iii) Waterproof suit, coveralls or a full-length apron.
1.3.3.4.2 Housekeeping/Chlorine Storage
(a) Use signs to clearly identify all areas where chlorine is used or stored. Only qualified personnel should be permitted to enter these areas,
(b) Do not store materials that may react violently with chlorine in the same room as chlorine. Put up visible warning signs prohibiting persons from taking these materials where the chlorine is stored,
(c) Do not store chlorine near busy roadways or where vehicles operate. Chlorine reacts with carbon monoxide to produce phosgene, an extremely poisonous gas,
(d) Store chlorine cylinders and containers in a cool, dry, and relatively isolated area, protected from weather and extreme temperatures:
(i) When storing cylinders and containers outside, shield them from direct sunlight,
(ii) When storing chlorine containers inside, store the containers in a well- ventilated building, away from any heat sources.
(e) Use cylinders and containers on a “First-In, First-Out” basis,
(f) Clearly tag or mark empty cylinders and separate them from full cylinders,
(g) Determine the most appropriate location for emergency equipment. Emergency equipment and a faucet should be available in a readily accessible location, but not inside the chlorine room because a worker (and emergency response staff) trying to use the emergency equipment or faucet during a chlorine leak risks further exposure,
(h) Store cylinders upright and secure them against tipping over and rough handling. Cylinders will discharge vapour when upright and discharge liquid when upside-down. Since chlorine gas tends to sink, provision should be made for low-placed ventilation near the floor that allows it to dissipate outward, as well as high-placed ventilation that allows the chlorine mist (the gas mixed with air) which tends to go upward, also to dissipate.
1.3.3.4.3 Handling Chlorine Cylinders
(a) Handle containers with care while moving or storing them. Do not drop or allow containers to strike objects,
(b) Use new gaskets as recommended by the chlorine supplier each time a cylinder or container is connected,
(c) Follow the chlorine supplier’s recommended disposal procedures for leaking containers. Do not modify, alter, or repair containers and valves. Only the supplier should carry out these tasks,
(d) Ensure that cylinders have valve protection hoods in place when not connected to a system,
(e) Do not lift a cylinder by its valve protection hood. The hood is not designed to carry the weight of a cylinder,
(f) If possible, open valves by applying a steady force to a 200 mm (8 in) wrench, without applying an impact force and without using an extension on the wrench. If this does not work, apply a light impact force by smacking the wrench with the heel of your hand,
(g) Do not use a wrench longer than 200 mm (8 in) to open or close valves. To prevent valve damage that could cause leaks do not use tools such as pipe wrenches or hammers. Valves on cylinders are designed to deliver full volume after one complete counter clockwise turn. Valves may be damaged if turned beyond this point. Immediately return containers with damaged or inoperable (but not leaking) valves to the supplier,
(h) If the valve is very difficult to open, loosen the packing nut slightly. Tighten the packing nut after the valve is opened or closed.
1.3.3.5 Electro-chlorinator
Chlorine is instantly produced by electrolyzing brine solution. Common salt is mixed with water to prepare brine solution. This solution is passed through an Electrolyser of electrodes comprising of anodes and cathodes, which are energised by D.C. current to produce NaOCl. This solution of sodium hypo chlorite is used as disinfectant. Basically, the electro chlorinator set comprises of two compartments; one comprising of brine solution tank, electrolyser, cooler, etc. and the other comprising of compact panel board (rectifier). Normal life of electro chlorinator is 12 years provided reconditioning of the electrodes at regular interval of four years is carried out.
1.3.3.6 Other Disinfectants
The other chemical based disinfectants generally in use are ionized silver coating, gaseous chlorine, ozone, chloramine, potassium permanganate and hydrogen peroxide. Alternatives to chemical disinfection, such as UV irradiation, are also being used for disinfection of drinking water.
1.3.3.7 Ozonation
Ozone is very strong oxidiser and powerful disinfecting property. Avery small concentration of ozone in water makes it free from bacteria, virus and pathogen much faster and with lesser concentration in a most effective manner. Ozone, an allotrope of oxygen having three atoms to each molecule, is a powerful oxidizing and disinfecting agent. It is formed by passing dry air through a system of high voltage electrodes. The major elements of an ozonation system are:
(a) Air preparation of oxygen feed,
(b) Electrical power supply,
(c) Ozone generation usually using a corona discharge cell consisting of two electrodes,
(d) Ozone context chamber, and
(e) Ozone exhausts gas destruction.
Advantages of ozonation system is that it requires shorter contact time and doses than chlorine, ozone does not directly produce halogenated organic materials unless a bromide ion is present. Limitations of ozonation system, ozone gas is unstable and must be generated onsite. A secondary disinfectant, usually chlorine, is required because ozone does not maintain an adequate residual in water.
1.3.3.8 Operation and Maintenance for Ultra-filtration, Micro filtration and Nano filtration
Basically, ultrafiltration and microfiltration are the protection mechanism of nanofiltration and these should precede Nano filtration, for more details refer Volume I. Because application of membrane filtration technology is still rare in Tanzania at the moment, the current edition of the manual refers designers to the manufacturers of membrane filters for all design specifications sample websites of the manufacturers are provided at the end of this DCOM Manual .
Previous Page: Part C: Operation and Maintenance of Water Treatment << >> Next Page: Chapter Thirteen: Treatment for Special Water Sources